EXTRA CREDIT - EXPERIMENT G
ELECTROCHEMISTRY ACTIVITY OF METALS
INTRODUCTION
The objective of this experiment is to develop an abbreviated activity series of metals using:
1. Displacement reactions
2. Relative reduction potentials.
BACKGROUND
A ranking of activity is called an activity series. A metal is more “active” than another metal if the first metal is oxidized by the second. In this experiment this means that if the solid metal reacts with the aqueous metal ion, the solid is more active. For example, when metallic iron (solid Fe) is placed in a lead solution (aqueous Pb2+), iron loses two electrons to form the Fe2+ ion (oxidation) and the lead will gain two electrons to form Pb solid (reduction).
Reduction: Pb2+(aq)
+ 2e- ® Pb(s) Fe(s)
Fe2
Oxidation: FePb(s)Pb2
®
Fe2+(aq)
+ 2e-
Overall reaction: Pb2+(aq) + Fe(s) ® Pb(s) + Fe2+(aq)
The opposite reaction, Pb(s) with Fe2+(aq), does not happen
Pb(s) + Fe2+(aq) does not make Pb2+(aq) + Fe(s)
Since Fe reacts with Pb2+, iron is more easily oxidized and therefore is more active than lead. A simple color change, bubbles, or any other observation signifies that the reaction is taking place. So Pb2+(aq) + Fe(s) would have some sort of observation while Pb(s) + Fe2+(aq) would not.
In part two of this experiment, simple metal to metal ion half-cells will be used in conjunction with a reference metal half-cell to establish voltaic cells. The potentials of the voltaic cells will be measured with a voltmeter. Knowing the overall cell potentials and arbitrarily assigning a value of zero to the reference metal half-cell, the relative reduction potential of each half-cell can be determined. The activity series established by the displacement reactions will then be compared to the reduction potentials to finalize the abbreviated activity series for the metals used in this experiment.
Standard half cell potentials, x°, are measured with a H2 (g) reference electrode. The voltages for the metals in this experiment, plus some extra ones, are listed below.
Table G-1. Standard reduction potentials of some metals.
Half reaction Voltage
Mg2+(aq) + 2e- ® Mg(s) -2.37 V
Zn2+(aq) + 2e- ® Zn(s) -0.76 V
Fe+2(aq) + 2e- ® Fe(aq) -0.44 V
Pb2+(aq) + 2e- ® Pb(s) -0.13 V
2H+(aq) + 2e- ® H2(g) 0.0 V
Cu2+(aq) + 2e- ® Cu(s) +0.34 V
Ag+(aq) + e- ® Ag(s) +0.88 V
Notice how in all reactions the metal ion is being reduced.
To find the overall potential of a reaction, two half reactions can be added together like Hess’s law. The only difference between Hess’s law and potentials is that the voltage does not change in value. Potentials only change in sign,
Fe2+(aq) + 2e- ® Fe (s) -0.44V
2Fe2+(aq) + 4e- ® 2Fe (s) -0.44V
2 Fe (s) ® Fe2+(aq) + 2e- NOT: 2 x (-0.44) or -0.88V
Fe (s) ® Fe2+(aq) + 2e- +0.44V
Iron’s reaction
always has the same value (voltage number) if the same equipment is conditions are used.
For the above example, the reaction of iron solid to iron (II) has a voltage of
0.44 V. This is using the
standard reference potential, H2 (g).
So for a reaction of Fe and Pb, the cell voltage would be,
Oxidation: Fe(s) ® Fe2+(aq) + 2e- + 0.44V
Reduction: Pb2+(aq) + 2e- ® Pb(s) ________ - 0.13 V
Overall reaction: Pb2+(aq) + Fe(s) ® Pb(s) + Fe2+(aq) + 0.31V
if the reference half cell is where oxidation takes place in this case with the more active metal (Fe). If the reduce metal is used as a reference, then the probes measure the same value but in the opposite direction (opposite sign). A positive voltage for a total reaction (not a half reaction) is spontaneous, so most reactions we observe in nature have a positive voltage. If the opposite reaction takes place the reaction voltage would be negative. A voltage would have to be applied to the cell for this “electrolytic” reaction to take place.
EXPERIMENTAL
Chemicals and Reagents:CHEMICALS
AND REAGENTS
1.0 M Copper Nitrate, Cu(NO3)2 HC
((<1032>))
1.0 M Magnesium Nitrate, Mg(NO3)2 HC
((<2032>))
1.0
M Zinc Nitrate, Zn(NO3)2 HC ((<1032>))
1.0
M Lead Nitrate, Pb(NO3)2 HC
((<3013>))
1.0
M Sodium Nitrate, Na(NO3) HC (<(1031)>)
0.1 M Silver Nitrate, Ag(NO3)2 HC
((<2002)>)
0.1
M Hydrochloric acid, HCl HC ((<2022)>)
6
M Hydrochloric acid, HCl HC (<(3023)>)
Small piecesparticles for part I
and flat pieces for part II of copper, lead, magnesium, zinc.
Equipment:EQUIPMENT:
Spot plate, hot plate, computer and voltage probe, filter paper to fit petri dish, petri dish, and spatula.
Safety:SAFETY CONCERNS:
Risk assessment is low to moderate since some of the solutions used in this experiment are toxic. Avoid contact and wash your hands when you are done with the experiment. Place used metals and solutions from PART ONE in the bottle marked " Waste 10% Cu, 10% Zn, 10% Pb, 10% Mg, 5% Ag, 20% Nitrate, 35% Water”. Metals used in PART TWO should be rinsed and returned to the correct original container.
Part 1 -- Displacement Reactions (Put metals in the waste jar in the fume hood.)
1. Generating the activity series with 1.0 M metals.
1. Clean a 12 – hole porcelain spot plate, a spatula, and a petri dish.
2. Like Figure G-1 below, place 1-4 pieces of a metal into each hole (depression) in the porcelain spot plate.
3. 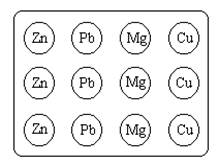
4. Figure G-1. Placement of solid metals onto the spot plate.
5. Check for oxidation on the metals. The metal will have a different coloration on and below the surface. Scrape the oxidation off a portion of the metal surface with a spatula.
a. Why? This is to remove any oxidized surfaces for the reaction to take place. Remember that you are trying to oxidize the solid metal with the aqueous one.
6. To each hole, add two drops of a solution with a different metal than the solid. A solid metal, like Cu, will not react with its soluble counterpart, Cu2+. As each solution is placed on a solid metal, record any observations, like a darkening of the metal’s surface, color change, etc. See Figure G-2:
n
7. 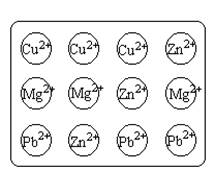
8. Figure G-2. Placement of aqueous metals onto the spot plate.
9. Put this on a hot plate and set heat at low or 2. (Each hot plate is different)
10. Observe the metals for five minutes while heating for any additional changes.
11. Remove the porcelain spot plate from the hot plate to cool.
12. Dispose of all metals and solutions in the waste container labeled "Waste 10% Cu, 10% Zn, 10% Pb, 10% Mg, 5% Ag, 20% Nitrate, 35% Water”. This is in the fume hood. Create an activity series while waiting for the porcelain plate to cool.
How do I create an activity series? To create an activity series, you must compare what solids reacted with what solution for each hole. The more active metals will react in the solid form, but not in the liquid form because they are more easily oxidized. (Oxidation happens from the solid form.) For example, metals A, B, and C react below:
A(s) + B2+(aq) ® A2+(aq) + B(s) (obs) A(s) + C2+(aq) ® no reaction (no obs.)
B(s) + A2+(aq) ® no reaction (no obs.) B(s) + C2+(aq) ® no reaction (no obs.)
C(s) + A2+(aq) ® C2+(aq) + A(s) (obs.) C(s) + B2+(aq) ® C2+(aq) + B(s) (obs.)
Since metal A(s) was oxidized by (reacted with) B2+, it is more active than B (A > B). Metal A(s) was not oxidized by C2+ and so the opposite is true. Metal C is more active than metal A. (C > A)
Metal B(s) was not oxidized by eitherA2+ or C2+, therefore both metals A and C are more active than B
(A > B and C > B)
With just these four reactions we can construct an activity series of the metals A, B, and C.
C > A > B
The last two reactions confirm this statement. C is oxidized by both A2+ and B2+, so it is more active than both metals A and B (C > A and C > B).
2. Additional activity series with Ag(NO3). Also a qualitative activity series with HCl.
1. Put the solid metals on the porcelain spot plate like in Figure G-1. Remember to put a few pieces in each depression. Solutions will be added as noted in Figure G-3 below.
2. To the top row of metals, add two drops of 0.1 M AgNO3 to each. Record any observations for each (silver metal is not provided because of cost). Is Ag more or less active than Cu? ________Pb?_______ Mg?________ Zn?_________.
3. To the middle row of metals, add two drops of 0.1 M HCl to each depression. Record observations for each.
4. To the same middle row, add two drops of 6.0 M HCl to each hole. Record as before.
5. Note: Unknown: The unknown for this experiment is one of the metal salt solutions (Pb(NO3)2, Cu(NO3)2, Zn(NO3)2, or Mg(NO3)2). In addition, AgNO3 is a possible unknown.
6. To the last row, add two drops of the unknown to each depression. Record as before. Your unknown will behave like one of the solutions above (including AgNO3) and therefore can be identified. If it is not possible to tell the difference between two metal salts based on the spot tests report both of them on the unknown report sheet.
7. 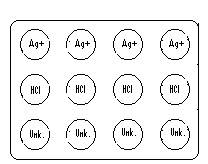
8. Figure G-3 Placement of AgNO3, 0.1 & 6M HCl, and Unknown onto spot plate.
9. Place the used metals in waste container labeled "Waste 10% Cu, 10% Zn, 10% Pb, 10% Mg, 5% Ag, 20% Nitrate, 35% Water”. Rinse the spot plate and return it to reagent bench.
The identification can then be confirmed (except for Ag) by measuring its potential in part two below.
Part 2 -- Relative Reduction Potentials. Four or five electrochemical cells can be made using a piece of filter paper, sodium nitrate, 4 or 5 flat pieces of the metals, and their nitrate salt solutions.
Note: Metals should be washed and put back in their reagent jars.
A. Making the Cells
1. Obtain a piece of filter paper small enough to fit in the bottom of a Petri dish. Cut the filter paper as shown in Figure G-4 and place in the Petri dish. The shape does not have to be exact.
2. Obtain the Cu, Mg, Zn, and Pb metal pieces from jars for part II. Wash and dry them.
3. With the edge of a spatula or a file scrape each side of the metals enough to have shiny spot.
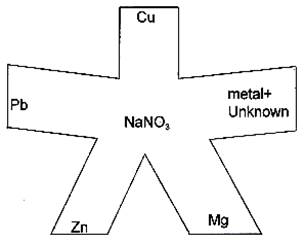
4. 
5. Figure G-4 Filter paper cut out for the electrochemical cell.
6. Preparing the voltage program to collect relative reduction potentials
a. Check to make sure both voltage probe leads are connected to Port 1. (red and black.)
b. Open. (Double click “CHM152-G Electrochem” on the desktop. No calibration is needed.)
c. If need be, adjust the x-axis on the graph to 300 seconds.
7. Place 1-2 drops of the metal nitrate solutions (1M) on the ends of the filter paper as shown in Figure G-4. Make sure that the metal nitrate solutions don't overlap. If the unknown was determined to be AgNO3 from the spot test, do not place a drop of it on the filter paper since we have no silver metal available to make the half-cell.
8. Place 1-4 drops of 1M NaNO3 in the center of the paper to form a salt bridge between the half-cells on the ends of the arms. Be sure the NaNO3 solution comes in contact with all the other solutions. If not, add another drop or two of solution so that it does. Do not let the paper dry out before testing the potentials or cell voltages.
i. Why do we use NaNO3? NaNO3 is put in the middle of our electrochemical cell to create a salt bridge. The salt bridge allows electrons to easily flow from the one to the other metal.
9. Quickly place the pieces of metal on top of their respective solutions (e.g. Cu(s) on Cu2+(aq)). Make sure the shiny part of the metal has good contact with the wet filter paper. Keep the topside of the metal dry. On top of the unknown, put the metal the unknown is suspected to be from the displacement reactions. Quickly go to the next part.
B. Graphing the reduction potentials from Cu and Pb relative standpoints.
Data Collection (relative reduction potential collection)
- Place the red probe firmly on the copper metal in the petri dish (Push down on the lead to expose it’s contact).
- Click “Collect.”
- Place the black probe firmly on the Pb metal for 20 to 30 seconds
- After 20 to 30 seconds, remove the black probe for 10 seconds. If the voltage does not quickly return to zero touch the ends of the probes together.
- Repeat the two steps above for Zn, Mg, and the unknown. If your unknown is silver (Ag) skip the voltage test for it. If your unknown is one of the other metals, the peak should be at the same height as that metal.
- Switch the reference (red) contact to Pb and measure the Zn potential.
- Click “Stop”.
Adjusting the graph
1. Adjust the scale on the time axis and voltage axis if needed to fit the data. Start by clicking on the first or last number on the axis scale, enter the new number, and press "return". Make sure the scale is easy to use (i.e. whole numbers!).
2. Edit the Graph Title to include your name, CHM152L, your section letter, date, experiment description or trial #, and other information.
3. Select "File" again and click on "Print Screen". Save the trial on your Z drive.
4. Identify each potential peak with the two metals in the cell (i.e. Cu-Pb is the first one).
5. Record the cell potentials measured on the graph in your lab notebook.
6. Trim and tape this graph into the lab notebook and have your TA sign and date it.
Clean up
1. Wash, rinse, and dry the metal squares and return them to the reagent bench. (Put in containers for part II returned metals!) Throw the filter paper in the trash.
- Rinse out the petri dish with tap water and return it to the reagent bench.
- If you have time try making other batteries (what you did above) out of half cell combinations and measure their voltages. See TA for more information.
ELECTROCHEMISTRY – EXTRA CREDIT
CHM 152L REPORT SHEET FOR UNKNOWN G-XXXX
STUDENT'S NAME_____________________________________ Dana ID__________
SECTION LETTER____ LOCKER #_____ DATE_________ UNKNOWN # G- .
TEACHING ASSISTANT___________________ INSTRUCTOR_________________
This report sheet should be turned into the chemistry stockroom. Do not write the hazard code, which has the form HC-xxxx, for the unknown number. The unknown number can be found on the vial containing the unknown at the top of the label or, if the vial is missing, the unknown number can also be found in the blue book for your lab section in the chemistry stockroom.
PART I - DATA FROM SPOT TESTS:
Rank the metals in order of activity from the spot test observations including Ag and explain your logic:
Identity of them Unknown Metal Solution__________________________________
PART II – MEASUREMENT OF POTENTIALS:
Annotate the potential graph with the potential (voltage) of each peak along with the half reaction. Staple the graph to this report sheet. Generate an order of metal activities based on potential:
POSTLAB QUESTIONS (answer on back of this sheet or attached sheet of paper):
- Do the two activities series support each other? Explain your answer using results.
- Calculate the Pb-Zn half cell from your Cu-Zn and Cu-Pb cells and compare to the one you measured.
- Calculate the four cell potentials measured using standard electrode potentials from Table G-1 and compare to ones you measured. Do they differ? Why?