CALORIMETRY – EXPERIMENT A
ENTHALPY OF FORMATION OF MAGNESIUM OXIDE
INTRODUCTION
This experiment has three
primary objectives:
1.
Find the heat capacity (Cp) of a
calorimeter and contents (calibration).
2.
Determine the DHrxn, the enthalpy of reaction, for several different
reactions, including the reaction of an unknown with a solution of HCl.
3.
Calculate the DHf, the enthalpy of formation, of MgO using Hess’ Law.
We will assume that the
energy exchanged between the calorimeter and the surroundings during and
following the reactions is small and at a slow, constant rate. You will become
familiar with calorimetry as this experiment is completed.
BACKGROUND
Calorimetry measures the energy that a reaction
produces or consumes. For example, the major difference between gasoline grades
is the octane number. Unleaded gas has an octane of 86, while Super Unleaded
gas has a higher octane. Calorimetry could be used to measure the heat or
energy produced when gasoline is burned. More heat (energy) would be produced
by the super unleaded gas so it would have a higher enthalpy compared to just
unleaded gas. Calorimetry could be used to see if a gasoline station is selling
the grades of gasoline it advertises.
The calories in food have
also been measured by calorimetry (hence the term calories). Usually
this is a measurement of calories (cal) per gram of food. Remember that
calories are easily convertible to joules (J) and grams can be converted to
moles if it is a pure chemical.
Enthalpy, represented by the symbol H, is a property
chemists use to describe the heat flow into or out of a system in a
constant-pressure process. This is often the case since most processes that are
carried out are exposed to the atmosphere as are the reactions carried out in
this course. The enthalpy of a reaction, DH, is
defined as the difference between the enthalpies of the products and the
enthalpies of the reactants. In other words, it is the change in energy for a
given amount of a given reaction. The enthalpy of formation, DHf
is defined as the enthalpy or heat change that results when one mole of a
compound is formed from its elements. The standard enthalpy of formation
is defined as the enthalpy of formation measured at 1 atm such that the
elements are in their standard state.
If a reaction is exothermic,
heat will be released, and the temperature of the system or reaction mixture
will rise. (In this experiment the heat and temperature rapidly increase and
then slowly decrease as heat is lost to the surroundings.) For endothermic
reactions heat will be absorbed or used and the temperature will decrease. In
this experiment we will use the experimentally measured enthalpy of reaction
for a series of exothermic reactions and Hess' Law to determine the heat of
formation for magnesium oxide (MgO). We will also determine the enthalpy of
reaction for an unknown metal oxide with an acid.
The enthalpy of reaction, DH, can be calculated using the equation:
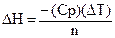 A-1
A-1
Where n is the moles of limiting reagent, DT is the change in temperature of the
calorimeter’s contents, and Cp is the heat capacity of the calorimeter. The
value for n can be determined knowing the amounts of starting material. The DT for a reaction can be calculated using the
temperatures before and after the reaction or the initial and final
temperatures. The heat capacity, Cp, of the calorimeter has to be
experimentally determined by doing a reaction where the DH is known. The heat capacity of the calorimeter is primarily
due to the solution in the cup.
Heat capacity (Cp) has units of kJ/°C. Physically, this means that it takes the value of the Cp in
energy to raise the calorimeter by 1°C.
For example, if a calorimeter has a Cp of 0.200 kJ/°C, the calorimeter, including its contents, must absorb 0.200
kJ of energy to increase 1°C. A 20 kJ/°C calorimeter increases 1°C with one hundred times more energy, or 20
kJ. Cp is a relative term to describe how much energy is needed to change the temperature
of a substance or system. The Cp of an ocean is huge (compared to a drop of
water). The heat needed to change the drop of water by 1°C could not change the ocean by 1°C.
The oceans of the world maintain the earth at temperatures that support life.
In this experiment, the calorimeter is defined as two
nested styrofoam cups, the lid, magnetic stir bar, and the temperature probe
tip, plus the 60.0 mL of the reaction mixture (mainly water). In order for the
heat capacity of the calorimeter to remain constant, all of these must be
present.
Figure A-1
Calorimeter Apparatus (ignore B-1)


NOTE: If less than 60 mL of reaction mixture was added, it
would take less energy to increase the calorimeter and contents by 1°C. In other words, the heat capacity would decrease. If
more than 60 mL of the mixture was added, more energy would be needed to
increase the calorimeter and contents by 1°C. The
heat capacity is then increasing.
Most importantly the volume of reaction mixture must
remain constant because of the large heat capacity of water. We want the Cp
to remain constant because it is the standard by which we can calculate unknown
DHrxn values in reactions 1,
2, and 4. The Cp is determined in the reaction of HCl with NaOH, using the
known enthalpy (energy/mole) for a strong acid/strong base reaction:
H+ (aq) + OH- (aq)
® H2O (l); DH = -55.90 (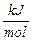 )
at 25°C A-2
)
at 25°C A-2
Rearranging equation A-1 we can use this DH to solve for Cp.
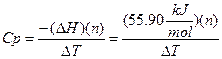 A-3
A-3
A value for DT can
then be determined for a known amount of moles (n). Once the Cp is known we can
use it to calculate DH for other
reactions where DT has been
experimentally determined. Please look in your textbook under calorimetry or
thermodynamics for more information on these concepts. The explanation to
determine DT is in the experimental
section below.
EXPERIMENTAL
This experiment essentially has three parts. In the first
lab period, the data to determine the enthalpy of reaction for Mg + HCl and MgO
+ HCl will be collected (one trial on each). During the second lab period, data
will be collected to calculate the Cp using the reaction of NaOH with HCl (two
trials). Also, the enthalpy of reaction for the Exp. A unknown reacting with
HCl will be determined (two trials). The trials for the Cp and unknown should
be run in the same lab period.
Be sure to label each graph carefully with your name,
section, date, reaction & trial, mass of reactant, etc. Note that the DH is not done until after Cp is determined
the second week. Tape graphs into your notebook as you print them.
EQUIPMENT AND MATERIALS:
1. Temperature
probe connected to computer via analog to digital interface box
2. Vernier
Data Logger software
3. Calorimeter
(two nested styrofoam cups and lid labeled with your bin number)
4. Thermometer
5. Stirrer-hot
plate and teflon stir bar
6. Spatula
and electronic balance
7. 25
mL graduated cylinder (or 25 mL pump dispenser)
8. Various
sizes of beakers and erlenmeyer flasks
9. 10
mL volumetric pipet or 10 mL pipettor
10. Wash bottle filled with RO
water
CHEMICALS:
1. (3033)
HCl 3.0M Hydrochloric acid - about 200mL needed
2. (3034)
NaOH ~5.0M Sodium Hydroxide - see carboy for exact concentration, about 30 mL
needed
3. (0220)
Mg Magnesium turnings - about 0.20g needed
4. (1011)
MgO Magnesium oxide - about 1.0 g needed
5. (1012)
Unknown A-xxxx in your unknown packet - need at least 2-3 grams
SAFETY CONCERNS:
Risk Assessment-Moderate to High (due to corrosive
liquids)
1. The
HCl and NaOH are corrosive. The unknown and MgO are mildly corrosive and some
are powders so avoid contact with solids and dust. Avoid contact, wear eye
protection at all times when working with these chemicals, and wash hands after
handling them. Do not rub your eyes when using these chemicals. Any small
spills should be cleaned up immediately with a damp sponge.
2. Any
contact with HCl or NaOH should be rinsed for 15 minutes with water.
3. All
solid and liquid chemical waste should be disposed of in the “Corrosive
Liquids” container.
EXPERIMENTAL PROCEDURE:
The Determination of DH of Mg with HCl and MgO with
HCl
FIRST LAB PERIOD
NOTE: If these links are
not present, click on the icon, "Vernier Programs" and then “Data
Logger”. Change the time to 400 seconds.
Calibration of the Temperature Probe:
Startup procedure
n
Select “Start”, “Programs”, “Chemistry Applications” and then
click on “CHM152L-A Calorimetry”.
Calibration procedure
n
Find your thermometer.
n
Fill one beaker with a slurry of ice. The beaker need not be
clean. (Fill with ice and put the minimum amount of water to make ice slush.)
n
Fill another beaker with hot tap water. The beaker need
not be clean.
n
Put the temperature probe tip and thermometer bulb together so
they touch and place them into the hot beaker and let sit for one minute. The
NOTE: Use the same
calorimeter and computer every lab period if possible.
NOTE: Only one trial is
needed for this reaction. Avoid adding extra heat from hands, hot
plate-stirrer; make sure the hot plate is turned off.
Why should you measure the
mass of Mg this way?
temperature can be read below
the graph. You do not need to click on the collect button. Record the
temperatures. If the temperatures are not within ±
0.5°C, see your TA.
n
Put the temperature probe and thermometer into the cold beaker
and let sit for one minute. You do not need to push the collect button. Record
the temperatures. If the temperatures are not within ± 0.5°C, see your TA.
n
Check the calibration again at the beginning of the second lab.
Mg Reaction. Determining the Enthalpy for Reaction (DH) of Mg with HCl (H+):
Mg(s) + 2 H+(aq)
® Mg2+ + H2(g)
n
Clean a teflon stir bar, 50 mL beaker, 25mL and 50mL graduated
cylinder and wash bottle.
n
Add 25.0 mL of 3.0 M HCl (use pump dispenser, ok to check volume
with 25mL graduated cylinder) and 35.0 mL of RO water to the calorimeter.
n
Put the temperature probe in through the lid and place the lid on
the calorimeter. Secure the probe with a clamp so that its tip is in the water but
away from the stir bar and off the bottom of the calorimeter (see fig.A-1).
n
Stir the solution with a teflon stir bar. (Do not heat.) Set
stirrer so the solution is mixed vigorously but slow enough so that it is not
splashed.
n
Put between 0.15 to 0.20 g (not 1.5) of Mg metal turnings into a
clean, dry 50 mL beaker. Record both the beaker’s mass and the mass of
the beaker and Mg.
n
Click the "Collect" button at the top of your screen to
start graphing the temperature. Do not add Mg turnings yet.
n
After about one minute, add the metal Mg turnings without
removing the temperature probe from the solution. (Crack the lid open, add Mg (s),
and then close the lid, it is ok if residual Mg sticks to the inside of the
beaker since you will reweigh it later to see how much Mg was added to the
calorimeter).
n
After about 10 seconds, briefly swirl the solution (holding the
cup in your hand) to dissolve any Mg left on the sides of the calorimeter. Do
this again after 60 seconds.
n
Reweigh and record the mass of the beaker that contained the
Mg turnings. Subtract this mass from the mass of the beaker and Mg to get the
mass of Mg used.
NOTE: See your TA if you
stop gathering data before you reach 350-400 seconds. Do not move the
calorimeter or delete or stop the program! Get help from your TA! You can also
hit “Collect” and then “Append to latest” to restart collecting data.
n
Continue graphing data until a linear line (part 3 in Figure A-2)
is made. (At about 350 to 400 seconds.)
n
Adjust graph scale and then do a linear fit (refer to Figure A-3).
Generate line 2 by selecting the linear part of the graph and doing a linear
fit. Use the analyze tool to find the starting time of the reaction, that would
be x in y=mx+b to determine the final temperature. Using m, b, and x calculate
the final temperature (Tf). The next page graphically explains this
process.
n
Point the cursor at the flat part of the graph before the
reaction starts to determine Ti and subtract this from Tf
to determine the change in temperature, Tf - Ti = DT. Calculate these values for every graph
done for exp. A.
n
Label the graph by clicking on the graph title. Edit the title to
include your last name, experiment title-reaction Mg with HCl, date, section
letter, and exact mass of Mg used.
n
Save the graph on your “Z” drive, my documents, or a thumb drive
using a logical file name and print it.
n
Write the values for Tf, Ti, and DT directly on this printed graph.
n
Clean and dry the calorimeter, temperature probe, and the 50 mL
beaker.
Explanation of the Graph: Below is a general
temperature vs. time graph representative for all reactions trials done for
this experiment. (Figure A-2) It is divided into three parts.
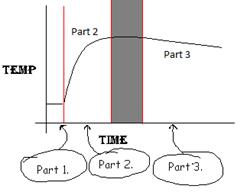
NOTE:
the dark band between Part 2 and Part 3 is a transition area that is not
usable for data analysis. The linear regression is done on part 3 to the right
of this dark area!
Figure A-2. A
general temperature vs. time graph
Part 1. This
is the initial temperature. Only one reactant is in the solution and so our
reaction is not happening. (For example, for Mg, only 25.0 ml of HCl and 35.0
ml of water are in the solution.) Between part 1 and part 2 the reactants are
mixed together.
Part 2. The temperature
is changing rapidly. Both reactants are now in the solution and are reacting to
give off heat. (For example, for Mg, this is because the Mg turnings are added
to the solution.) Somewhere within the blocked out region the reaction stops.
Part 3. The
reaction has already stopped. Since the calorimeter isn’t a perfect insulator,
heat is lost to the environment and, as a result, the temperature decreases.
The temperature should be constantly decreasing. (For example, for Mg, all of
the Mg has been converted to Mg2+.)
How do you get DT? DT is equal to the extrapolated final
temperature minus the solutions initial temperature. So DT = Tf – Ti. Below is the same general
graph from figure 1, but it has been extrapolated to find Tf (Figure
2). Tf can also be determined by doing a linear regression on the
linear, right hand side of the curve to determine the slope and y-intercept of
line 2 and then solving for the temperature using the time at the start of the
reaction (line 1).
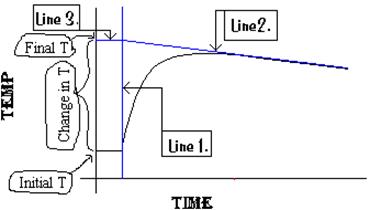
Figure A-3. Extrapolation
of a Temperature vs. Time Graph to Find DT.
Line 1. This
line represents the time when the reactants were mixed and so the start of the
reaction.
Line 2. This
line helps us model what the final temperature would be if the reaction and
temperature measurement were instantaneous. It compensates for the heat lost
from the calorimeter so that we can estimate the final temperature if the
reaction and temperature measurement were instantaneous. This line is important
because it compensates for heat lost to the environment while temperature is
measured during and after the reaction.
Line 3. This
line is drawn at a right angle to line 1 to intersect the point where lines 1
and 2 meet. It is there to help read the final temperature, Tf, at
the y-axis.
Calculations:
IMPORTANT: For nearly
all calculations in this manual the value you are calculating will be in bold
print.
In this reaction you were trying
to find the DH for reaction of Mg with
HCl. Unfortunately all of the calculations cannot be done until the Cp in
equation A-1 is found after doing the NaOH reaction. The limiting reagent
is Mg, so find the moles of Mg.
 A-4
A-4
where g Mg is grams of Mg and MM
of Mg is the molar mass of Mg (g/mol). Now n is found.
Find DT by extrapolation on the printed
graph as shown in figure A-3. Draw all lines with a pen and a ruler. Label Ti
(initial), Tf (final), and DT
on your graph
NOTE: Only one trial is
needed for the MgO reaction.
NOTE: Never click on “New
Graph” to start a new trial. Instead just close the program and then reopen it.
If you do click on “New Graph” the time scale will decrease to 200 seconds. Do
not move the calorimeter or delete or stop the program if it does stop early!
Get help from your TA!
.
MgO Reaction. Determination of the Enthalpy for Reaction of MgO with HCl:
MgO(s) + 2
H+ (aq) ® Mg2+(aq)
+ H2O(l)
n
Add 25.0 mL of 3.0 M HCl and 35.0 mL of RO water to the
calorimeter.
n
Clean the temperature probe by thoroughly spraying with your wash
bottle into a large waste beaker.
n
Put the temperature probe in the calorimeter as was done before
and vigorously stir the solution (but don't splash) with a Teflon stir bar. (Do
not heat)
n
Put between 1.0 to 1.2 g of MgO powder into a clean, dry 50 mL
beaker. Record the mass of both the beaker and the beaker with MgO.
n
If Data Logger is open close it and select “Start”, “Programs”,
“Chemistry Applications” and then click on “CHM152L-A Calorimetry”.
n
Click the “Collect” button at the top of the screen to start graphing
the temperature. Do not add MgO powder yet.
n
After about one minute, add the white powder MgO without removing
the temperature probe from the solution. (Crack the lid open, add MgO(s),
and then close the lid. Its ok if a residual amount of powder remains in the
beaker since it will be reweighed later to determine the amount transferred to
the calorimeter).
SECOND LAB PERIOD
NOTE: Check the calibration of temperature probe and
calibrate if needed.
Remember: 10.00 mL
is more precise than
25.0 mL. (cont.)
n
After about 10 seconds, swirl the solution to dissolve any MgO
left on the sides of the calorimeter holding it with your hands. Do this again
after 60 sec
n
Reweigh the beaker (with traces of MgO powder not transferred)
and subtract this from the mass of the beaker and MgO to determine the actual
amount of MgO transferred to the calorimeter.
n
Continue graphing data until a linear line (part 3 of figure A-2)
is made, then click on Stop at the top of the screen. (at about 350 to 400
seconds.)
n
Adjust scale of graph, do a linear fit, and find the DT as was done for Mg rxn.
n
Label the graph by clicking on the graph title. Enter in last
name, experiment title-Reaction MgO + HCl, date, section letter, and mass of
MgO used.
n
Save the trial on your “Z” drive, my documents, or a thumb drive
and print it.
n
Clean and rinse all glassware, the calorimeter and temperature
probe.
n
Before leaving, trim graphs to size and tape into the
notebook, and have TA sign and date notebook.
Calculations:
These are the
same calculations as described for Mg reaction. The calculations are now for
the reaction of MgO (s) with HCl (aq). The limiting
reagent is MgO so find the moles of MgO.
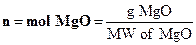 A-5
A-5
Now n (mol of
MgO) is found. Extrapolate your printed graph to find DT.
Determination of the Cp and DHrxn of an unknown
with HCl
NaOH + HCl
Reaction. Determination of Cp:
NaOH (aq) + HCl (aq) ® H2O (l)
+ NaCl (aq)
n
Clean a 25 mL graduated cylinder, 10 mL volumetric pipet, a
spatula, a 50 mL beaker, and a wash bottle.
n
Add 25.0 mL RO water and 10.00 mL of NaOH to the calorimeter and
measure its temperature.
(cont.) What kind of glassware
should you use for this? _______
_________________
NOTE: Never click on “New
Graph” to start a new trial. Instead just close the program and then reopen it.
If you do click on “New Graph” the time scale will decrease to 200 seconds. Do
not move the calorimeter or delete or stop the program if it does stop early!
Get help from your TA!
NOTE: Be sure to record the
exact NaOH molarity of the carboy.
NOTE: At least two Cp trials
need to be done. Avoid adding extra heat from your hands or hot plate.
n
Measure out 25.0 mL of 3.0 M HCl and an ice bath or hot water to
adjust its temperature so that it is within ±0.5°C of the NaOH solution. Do not get any of
the NaOH solution in the HCl solution. Rinse and dry your thermometer between
solutions.
n
Put the temperature probe in the calorimeter and stir the
solution with a teflon stir bar. (Do not heat)
n
Click the Collect button at the top of the screen to start
graphing the temperature probe. Do not add HCl solution yet.
n
After about one minute, add the 3.0 M HCl without removing the
temperature probe from the solution. (Crack the lid open, add HCl(aq),
and close the lid.)
n
After about 10 seconds, briefly swirl the solution.
n
Continue graphing data until a linear line (Figure A-2: see part
3 of this figure) is made. (At about 350 to 400 seconds.)
n
Adjust scale of graph, do a linear fit, and find the DT as was done before.
n
Label the graph by clicking on the graph title as before. Enter
your last name, experiment title, date, section letter, and NaOH with HCl,
Trial 1 or 2, and molarity of NaOH.
n
Save the run on your “Z” drive, my documents, or a thumb drive
and print it.
n
Clean and dry the calorimeter and temperature probe.
n
Repeat this once.
Calculations:
The calibration of the
calorimeter is now complete! Cp can now be calculated and used for all other
calculations! First solve for the moles of NaOH (limiting reagent).
(M of NaOH) * (L of
NaOH) = mol NaOH = n
Where M is molarity (exact
molarity on carboy) and L is liters of NaOH that was used. (You need to convert
from mL). Now n (mol of NaOH) is found. Plug this into equation A-3. Also the
enthalpy of neutralization (DH) of a
strong base by a strong acid is a constant –55.90 kJ/mol at
25°C. This is shown by reaction A-2.
H+ (aq)
+ OH- (aq) ® H2O
(l); DH = -55.90 (kJ/mol)
at 25°C A-2
With this value, the moles of
limiting reactant (n), and after determining DT
from your graphs by extrapolation, the equation A-3 becomes a simple plug and chug.
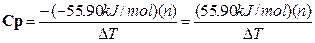 A-3
A-3
Repeat this calculation for the second trial
and use the average Cp value for the calculation of DH for both Mg and MgO. If the two Cp values differ by more than
±0.02 you may want to run a third trial
to determine a more precise value. Calculate the median of the Cp values. Now
the Enthalpy for Reaction (DH) of Mg
with HCl can be calculated using theDT
and moles of Mg previously calculated and plugging them into the equation below
using the Cp determined above.
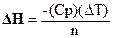 A-1
A-1
Now repeat this calculation using the data for MgO to
determine the DH of MgO.
Calculation of the Molar Enthalpy of Formation of
Magnesium Oxide, DHf:
Using Hess’ Law to combine the calculated DH values and a known DH (-285.8 kJ/rxn), the
DHf (MgO)
can be calculated.
Mg(s) +
2 H+(aq) ® Mg2+(aq)
+ H2 (g) DH=
___________
MgO(s) +
2 H+(aq) ® Mg2+
(aq) + H2O(l) DH= ___________
H2
(g) + 1/2O2 (g) ® H2O (l) DH= -285.8 (kJ/mol)
---------------------------------------------------------------------------------
Mg (s) +
1/2 O2 (g) ®
MgO (s) DHf=
___________
Example: Let say the DHrxn for the below equations was determined
experimentally.
A + 2B ® C + D DHrxn= -2 kJ/mol
2B ® 2C + 3D DHrxn = -5
kJ/mol
3A + 4B ® C DHf (C) = ________
When the first equation is multiplied by three, and the
second equation is flipped around, this equation becomes solvable.
3A + 6B ® 3C + 3D DHrxn= 3(-2 kJ/mol)
= -6 kJ/mol
2C + 3D ® 2B
DHrxn
= -1(-5 kJ/mol) = 5 kJ/mol
3A + 4B ® C DHf (C) = (-6) + (5) = -1 kJ/mol
Notice how chemical D and some of B and C cancel out
because they are on opposite sides of the first and second chemical equation.
NOTE: At least 2 trials
also need to be done for reaction of HCl with the unknown. Avoid adding extra
heat from hands, hot plate, etc
Enthalpy for the Reaction of HCl with an Unknown Metal
Oxide:
n
Add 25.0 mL of 3.0 M HCl and 35.0 mL of RO water to the
calorimeter.
n
Clean the temperature probe by thoroughly spraying with your wash
bottle into a 600 mL beaker.
NOTE: If your unknown is
sticky see your TA.
NOTE: The unknown metal
oxide is assumed to have a formula weight of 120.0 g/mol
!
NOTE: Never click on “New
Graph” to start a new trial. Instead just close the program and then reopen it.
If you do click on “New Graph” the time scale will decrease to 200 seconds. Do
not move the calorimeter or delete or stop the program if it does stop early!
Get help from your TA!
n
Put the temperature probe in the calorimeter and vigorously stir
the solution with a teflon stir bar but avoid splashing. (make sure the heat is
off)
n
Before using your unknown, make sure it is a powder. If it is
clumpy, grind it up in a clean, dry mortar and pestle. Put between 1.0 to 1.2 g
of unknown powder into a clean, dry 50 mL beaker. Record mass of beaker &
contents.
n
Click the "Collect" button at the top of your screen. Your
temperature probe will display the data it is collecting on the graph. Do
not add unknown yet.
n
After about one minute, add the white powder unknown without
removing the temperature probe from the solution. (Crack the lid open, add
unknown powder, and then close the lid. It is ok if a residual amount of powder
remains in the beaker since it will be reweighed later to determine the amount
transferred to the calorimeter)
n
After about 10 seconds, briefly swirl the solution to dissolve
any unknown left on the sides of the calorimeter. Do this again after 60
seconds.
n
Reweigh the beaker with unknown powder not transferred into the
calorimeter. Continue graphing data until a linear line (part 3 in fig A-1) is
made and then click on "Stop". (At about 350 to 400 seconds.)
n
Adjust scale of graph, do a linear fit, and find the DT as was done before.
n
Label the graph by clicking on the graph title. Enter in last
name, experiment title, date, section letter, Unknown with HCl, Trial 1 or 2,
& mass unknown.
n
Save the run on your “Z” drive, my documents, or a thumb drive
and print it.
n
Do a second trial after cleaning the cup and probe.
n
Clean and rinse all glassware and the temperature probe.
n
Before leaving, trim graphs to size and tape into the
notebook, and have TA sign and date notebook.
Calculations:
The unknown metal oxide is assumed to have a formula
weight of 120.0 g/mol. These are the same
calculations as described in the reaction of Mg. The calculations are now for
the reaction of unknown (unk(s)) with HCl (aq).The
unknown is the limiting reagent.
(g unk) / (120.0 g/mol
unk ) = mol unk = n
Now use the value for n (mol of unk), the DT determined from the graph, the Cp and the
equation below to calculate the enthalpy of reaction for the unknown with HCl:
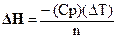 A-1
A-1
Calculate this value for each
trial and report the median value.
Be sure to complete a calculation
check before turning it in! When doing the calculation check, be aware that the
heat of reaction is the same as enthalpy of reaction and that these values are
negative numbers for exothermic reactions. Be sure to get a printout of the
calculation check. Fill out the report sheet for experiment A and staple the
printout of the calculation check to it. Turn the report sheet in to your TA
for grading. The report sheet for the unknown must be turned in by the next lab
period.
POST LAB WORK (In your
lab notebook):
1. Precision Calculations
a.
Calculate the
range of your two Cp trials.
b.
Calculate the
relevant percent range of your two Cp trials.
c.
Calculate the
range of your two Unknown ΔH trials.
d.
Calculate the
relevant percent range of your two Unknown ΔH
trials.
e.
Comment on both
relative percent ranges and relate these to the possible errors that you made
during the experiment.
2. Error Calculations
a.
Look up the
published (true) value for the DH°f , the Standard Molar Enthalpy of Formation for MgO(s), using your textbook or the internet.
b.
Calculate the
error, or difference, between your MgO ΔHf
and the published value.
c.
Calculate the
relative error for your MgO ΔHf
and the published value.
d.
Comment on this
relative error and relate it to the possible errors that you made during the
experiment.
ERROR ANALYSIS
1. List all experimental or procedural
errors that were observed. Look at relative percent ranges, relative percent
error, and the correlation coefficient for linear regressions to gauge where
there could be problems.
(What did go wrong?)
2. List all possible sources of errors
that could have occurred during the experiment.
(What could have gone
wrong?)
3. What could be done in order to
improve the results of the experiment?
(How to improve)
CALORIMETRY - EXPERIMENT A
CHM
152L REPORT SHEET FOR UNKNOWN A-XXXX
STUDENT'S
NAME_______________________ ID#_______________ Dana ID_____
SECTION______ WORKSTATION
#______ DATE______ UNKNOWN # A-_______
TEACHING
ASSISTANT____________________ INSTRUCTOR_________________
This report sheet should be
turned in to your TA. Do not write the hazard code, which has the form HC-xxxx,
for the unknown number. The unknown number can be found on the top of vial
label containing the unknown in the format A-xxxx. The unknown number can also
be found in the section blue book. Be sure to attach the calculation check
to this sheet before submitting for grading.
I. Calibration of Calorimeter
– Section Workstation # on Calorimeter_________
Cp Values ____________
______________
Mean or Average Cp
____________ kJ/oC (use median if more than two trials)
II. Enthalpy or Heat of
Reaction: Mg(s) + 2 H+(aq) ® Mg2+(aq) + H2(g)
____________
kJ/mol
III. Enthalpy or Heat of
Reaction: MgO(s) + 2 H+(aq) ® Mg2+(aq) + H2O(l)
____________
kJ/mol
IV. Enthalpy or Heat of
Formation MgO __________ kJ/mol
V. Enthalpy or Heat of
Reaction: Unknown Oxide + HCl
Values For Each Trial _________
___________
Mean or Average Value
____________kJ/mol For Unknown # A-_______
(use median if more than
two trials)
![]() A-1
A-1

![]() )
at 25°C A-2
)
at 25°C A-2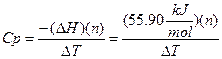 A-3
A-3
