| BIO 190: The Class: Chemistry of Life: Lesson 2 | ||||
| BIO 190: The Class: Chemistry of Life: Lesson 2 | ||||
Water is the most abundant compound in cells. Water has several unique properties that equip it for its essential role in living organisms.
Water has:
Water spontaneously ionizes to a slight degree. The pH of a solution describes the hydrogen ion concentration. The pH of distilled water is 7, which is considered neutral pH. Acids have pH values of less than 7 and bases have alkaline pH values of greater than 7. Try the interactive pH scale below which shows the pH values for some common substances.
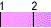 |
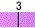 |
 |
 |
 |
 |
 |
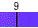 |
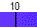 |
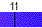 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
The term "organic molecule" is currently used in a broad sense to apply to compounds that contain carbon. The ability of carbon to form bonds with other carbon atoms in chains in a multitude of configurations allows great opportunities for complexity at the molecular level.
Examples of organic molecules include the following major categories.
Green plants from water and carbon dioxide synthesize carbohydrates. This process, known as photosynthesis, is one on which all life depends.
Carbohydrates are divided into three classes:
Lipids are fats and fatlike substances, characterized by low polarity.
As such they are insoluble in water.
Examples of lipids are:
Browse through the Protein Chemistry site below before proceeding. Also, be sure to open any links provides further down in the outline.
Proteins are large and complex molecules made up of 20 amino acids. (figure 2-13). Amino Acids
Proteins are highly organized molecules. Biochemists refer to them in terms of four levels of organization or structures. (figure 2-14).
-determined by the kind of sequence of amino acids
Browse the sites below for additional information.
Quaternary Structure
-used to describe proteins with more than one polypeptide chain unit.
The Role of Proteins as Enzymes
Although proteins perform many functions, their most important role is as enzymes. Enzymes are biological catalysts, which are required for just about every reaction in living things.
Chapter 5
Protein ChemistryNucleic acids are complex molecules that encode, via their nitrogenous bases, the genetic information that is the framework for all life. The two kinds of nucleic acids in cells are
-deoxyribose nucleic acid (DNA)
DNA Graphics Gallery-ribose nucleic acid (RNA).
These nucleic acids are made up of repeated units of nucleotides, each of which contains a sugar, a nitrogenous base and a phosphate group.
Once you have completed the lesson, you should go to Assignment 2-1.
E-mail the professor W. Sylvester Allred at Syl.Allred@NAU.EDU, or call (520)523-7214

Copyright 1997 Northern Arizona
University
ALL RIGHTS RESERVED