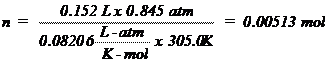CHM151L Extra Credit
Assignments:
There are three possible extra credit assignments possible:
- Titration of Household Products
- Molar Mass of a Metal (old experiment 7)
- Thermochemistry Experiment (old experiment 8)
Each experiment is worth a maximum of 20 points each. These
experiment should only be done once you have completed all the normal
experiments with a pass. In order to get full credit you must do excellent work
and have your data initialed by your TA in the same period the work was done
and this “raw data page” must be attached to the work. Good luck and have fun
doing the experiments listed below! Feel free to do any or all of them.
Titration of Household
Products (1-20 pts extra credit)
Comparison
of Common Household Products
In this experiment you will be given two different
brands of a household product to analyze for percent by mass of active
ingredient (see table below). Take your two smallest beakers to the lab prep
stockroom (216) where you will be assigned two brands of one product type to
analyze (assignment based on last digit of packet number). The standard NaOH
(about 0.1 M) prepared in Experiment 6 can be used for the STD NaOH if you have
enough left over. Otherwise the chemistry stockroom will provide you with
standard NaOH and HCl solutions that can be used as your titrant (both
solutions are approximately 0.2 M, the exact molarity will be provided). Take a
flask to the lab prep stockroom to get the standard solution you need and be
sure to record the exact molarity of the NaOH or HCl. Indicators will be
available in the lab prep stockroom or in the lab.
Do at least two titrations on each brand
following the instructions in the table below. Be sure to weigh out the
amount of product used for each titration to the nearest mg (even liquids).
The sample does not have to be the exact same weight noted and should be
adjusted in additional trials such that the volume of titrant used is between
20 to 24 mL if possible. Dissolve the sample in about 30 mL of distilled
water. If more than 25 mL of titrant is needed, take a final buret reading,
refill the buret, and continue the titration. The total volume would be the sum
of the volumes delivered. Calculate the percent by weight of active ingredient
for your product. Devise a report format similar to the report sheet for
Experiment 6, part C. Show all data collection and calculated values. Use
correct significant figures. Determine the percent by mass of active
ingredient for each trial and the median and the range for each brand.
The report for this extra credit should include a
one-paragraph introduction stating the experimental objectives, a
one-paragraph summary of your procedure, your results in a table format
described above, and a discussion in which you will interpret your results
(write a conclusion). Your work must be neat, understandable, and correct
for you to get full credit. In your conclusion be sure to discuss your
precision for each brand and why your work resulted in this kind of precision.
The range between trials for each brand could be used to estimate precision.
Comment on any sources of error or experimental problems and how they might
relate to your precision.
Household Products for Acid or Base Analysis and
Comparison
|
|
Active
|
Approx. Amt.
|
|
|
Last Digit of
|
|
Product
|
Ingredient
|
of Product to Use
for first trial
|
Indicator
|
Titrant
|
Packet No.
|
|
Drain cleaner
|
NaOH
|
0.15 g
|
Methyl Red
|
STD HCl
|
0 or 1
|
|
Ammonia cleaner
|
NH3
|
2 g (2 mL)
|
Methyl Red
|
STD HCl
|
2 or 3
|
|
Baking soda
|
NaHCO3
|
0.3 g
|
Methyl Orange
|
STD HCl
|
4 or 5
|
|
Toilet bowl cleaner
|
HCl
|
0.8 g (0.8 mL)
|
Phenolphthalein
|
STD NaOH
|
6 or 7
|
|
Vinegar
|
HC2H3O2
|
4 g (4 mL)
|
Phenolphthalein
|
STD NaOH
|
8 or 9
|
Molar Mass of a Metal
Experiment Seven: Extra
Credit
Introduction
You have been introduced to the concept of the
"mole" in lecture and have used this concept in previous
experiments. You have also become familiar with the concept of molar mass and
have used it as a conversion factor when calculating the number of moles
present in a given mass of a substance. So far, when you have needed a molar
mass (molecular mass or atomic mass for an element), you have been able to
obtain it by adding together molar masses from a table. However, this
procedure cannot be used when working with an unknown substance. The formula
of such a substance is, of course, unknown, and it is necessary to determine
its molar mass experimentally.
Gram molar mass or molar
mass is defined as the number of grams in one mole of a substance. Thus,
whenever the number of moles in a given mass of substance can be experimentally
determined, the molar mass of that substance can be calculated. For example,
if 0.0264 moles were found to be present in 3.69 grams of an unknown substance,
the molar mass of that substance could be calculated as follows:
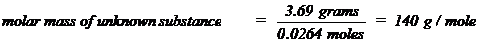
There are many ways to
determine the number of moles in a given mass of substance. If an unknown
substance is a gas, can be easily changed to a gas, or can produce a gas in a
reaction, the simplest method is to measure the volume of the gas. For ideal
gases, the volume does not depend on the type of substance present, but only
the temperature, the pressure, and the number of moles present. Thus, if the
volume of a given mass of gas at a known temperature and pressure can be
determined, the number of moles present in that quantity of gas can be
calculated.
Calculating
the Number of Moles in a Given Sample of Gas
The Ideal Gas Law, PV =
nRT, is an equation relating the pressure (P), volume (V), absolute temperature
(T), and number of moles (n) to each other. The value of the ideal gas
constant (R) depends on the units used in expressing the variables. The value
of 0.08206 liter-atm/K-mole is most often used and is appropriate when
indicating pressure in atmospheres, volume in liters, and temperatures in
Kelvin (absolute). Since volume is frequently measured in milliliters,
pressure in torr (mm of Hg) and temperature in degrees Celsius (ºC), these
quantities must be converted to the proper units before the Ideal Gas Law can
be used with R. The following problem illustrates the use of the Ideal Gas Law
to calculate the number of moles in a given volume of a gas at a specified
temperature and pressure.
Sample
Problem:
A sample of gas was collected and found to have a
volume of 152 mL at an atmospheric pressure of 677.7 mm Hg and a temperature of
32.0ºC. How many moles of gas are present? First the pressure must be
corrected for the vapor pressure of water since the gas was collected over
water. Use the information at the bottom of page 73 the vapor pressure of water
is 37.7 mm Hg at 32.0ºC so the corrected pressure (P) would be 677.7 – 37.7 =
642 mm Hg. The pressure, volume, and temperature are then converted to the
units of R and plugged into the ideal gas law, PV = nRT, rearranged to solve for
n, the number of moles:
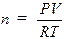
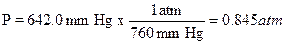
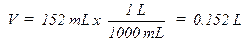
T (K) = 32.0º + 273 = 305.0 K (0ºC = 273K)
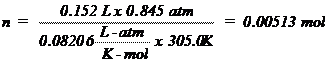
The molar
mass can be calculated by dividing the mass of the sample by the moles as was
done on page 71.
The Experiment
Risk
Assessment: Moderate to high when using the 6M HCl and producing hydrogen gas.
Moderate otherwise.
Note: There is a video on the
experimental procedure for this experiment on the CHM151L homepage that you
should view before doing this experiment.
Determination
of Molar Mass of Calcium and an Unknown Metal
The reaction of calcium with
water was studied in experiment 3. A gas was produced during the reaction.
You will carry out an experimental procedure to measure the molar mass of calcium
metal using the ideal gas law (PV=nRT) and the reaction of a known mass of Ca
with water. The moles of Ca can be calculated from the volume of hydrogen (H2)
gas collected using the ideal gas law. The moles of the two are equal because:
Ca +
H2O → CaO + H2
The molar
mass (in g/mol) can then be determined by dividing the grams of Ca used by the
moles of H2 produced.
Caution: Avoid handling the calcium metal with your
hands. Instead use crucible tongs or a test tube clamp. Keep any gas produced
away from open flames in case it is hydrogen (very flammable!) and vent this
gas in a fume hood. Wear goggles at all times in the lab.
Procedure to Determine Molar Mass of Calcium (be sure to
watch the video):
- Obtain a gas collection apparatus and a 100mL graduated cylinder for
experiment 7 from your workstation glassware locker and make sure it works
with a 6 inch test tube.
- Obtain and weigh a 0.05-0.07g piece or amount of Ca to a precision
of 0.001g and place in a clean, dry vial. Avoid touching Ca with bare skin.
- Place about 2mL of RO water in your six inch test tube.
- Fill a 100mL graduated cylinder completely with tap water and place
it up-side-down in a 600mL beaker that is about 2/3 full of water so no
air is in the graduated cylinder. This can be done in the tap water tub
for exp. 7 by the sink.
- Assemble the apparatus shown
below.

- Now tilt the test tube closer to a near horizontal position but keep
the liquid in the end of the test tube, remove the stopper, place the
metal in the tube, put the stopper back in to make a tight seal, and rock
the tube to get the metal in the RO water in the tube.
- Wait until the reaction is complete and record the temperature of
the water in the beaker and the atmospheric pressure with today’s date
from the board.
- Remove the gas collection apparatus from the graduated
cylinder/beaker.
- Now match the water levels in the beaker and graduated cylinder by
moving the graduated cylinder up and down until the levels match. Use the
4L beaker if needed to do this. Record the volume.
- Keeping the graduated cylinder in the 600 mL beaker take it to the
hood and release the hydrogen gas.
- The solution in the test tube should be placed in the corrosive
waste bucket.
- Subtract the vapor pressure of
water from the atmospheric pressure to correct for the water vapor present
inside the graduated cylinder. The vapor pressure of water is dependent on
water temperature. Use the table below, or for a more precise correction,
use the table on the board in the lab to determine the water vapor
pressure:
Vapor Pressure of Water
Temperature (degrees C): 15 16 17 18 19 20 21 22 23
Pressure mmHg: 12.8 13.6 14.5 15.5 16.5 17.5 18.6 19.8 21.1
Temperature (degrees C): 24 25 26 27 28 29 30 31 32
Pressure mmHg: 22.5 23.8 25.2 26.7 28.3 30.0 31.8 33.7 35.7
- Use the corrected vapor pressure to calculate the moles of hydrogen
present (see page 72 earlier in the lab manual). Use this and the mass of
calcium to calculate the molar mass of calcium in g/mol knowing that the
moles of hydrogen will be equal to moles of Ca that reacted. Compare this molar
mass to the one listed in the periodic table. Calculate the error and
then the percent error between the two molar masses and then answer the
questions.
Procedure to Determine the Molar Mass of the Unknown:
- The unknown for experiment 7 from your unknown packet will contain a
metal for which you will determine the molar mass (will not match periodic
table).
- Use the same procedure used for calcium except use the unknown
instead of the Ca and 6M HCl
Caution: 6M
hydrochloric acid is corrosive so wear goggles and rinse with water immediate
in case of contact with skin.
instead
of the distilled water in the test tube. Use a pump dispenser to place 2.0 mL
of 6.0M HCl (hydrochloric acid) in the six inch test tube. Dispose of the used
6M HCl in the corrosive waste bucket in the hood.
- Adjust the sample mass to maximize the volume collected, and/or
change the size of the graduated cylinder to provide greatest precision.
Repeat this procedure two more times with 2.0mL of fresh 6M HCl for each
trial. (hint: use ratios)
- Clean the apparatus and any graduated cylinders and return them to
your glassware workstation before the end of the lab period. DO NOT STORE
THEM IN YOUR LOCKER BINS! Doing so will cost you 5 points.
- Complete the calculations for each trial knowing they are the same
as for Ca except the reaction is slightly different:
M + 2HCl → MCl2 + H2
where
M is your metal unknown. Show all data and calculations on your report sheet.
So to
summarize the calculations for the molar mass of calcium or the unknown:
1.
n=PV/RT where R=Gas Law Constant of 0.08206 L-atm/°K-mol
2.
P=(Barometric Pressure on Board - Vapor Pressure Water) x 1 atm/760 mmHg
3.
V=(Volume Hydrogen Gas collected in mL) x 1L/1000mL
4.
T=Temperature of Water °C + 273
5.
Solve for n.
6.
Molar Mass=Mass sample in grams/n
The percent
error for Ca is
(measured molar mass Ca – true molar mass Ca) x
100%
True molar mass Ca
When
doing the calculation check for the unknown, use the corrected pressure for the
calculation check and be sure to enter values using the requested units.
Name____________________________________ Student
ID#_______ Dana ID_____
Lab
Section Letter___________ Locker #__________ Date_______________________
Extra
Credit - Experiment 7
Molar
Mass of a Metal
Experimental
Determination of the Molar Mass of Calcium
Outline Experimental Procedure:______________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
Risk Assessment:____________________________________________________________________
TA Signature and Date for Procedure
& Risk Assessment:_____________________________________
Data, Observations, and Calculations (must show all
data and calculations):
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
Molar Mass of Calcium (g/mol)________________________________________________________
True Molar Mass of Calcium From Periodic Table (g/mol)__________________________________
Difference Between Molar Masses (Error)_______________________________________________
Percent error in Molar Mass Measurement______________________________________________
What measurement(s) caused the greatest error in the
determination of the molar mass?
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
How could you improve your experimental procedure for
determining the molar mass of calcium?
_________________________________________________________________________________
_________________________________________________________________________________
Outline Experimental Procedure:______________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
_________________________________________________________________________________
Molar Mass of Unknown: Data and Calculations (must
show all data and example calculation):
Mass of Unknown Metal (g) ______________________________________
Barometric pressure (mmHg); don’t
use in calc. check ______________________________________
Vapor pressure water (mmHg); don’t
use in calc. check ______________________________________
Corrected Barometric pressure (mmHg) ______________________________________
Temperature of water (ºC) ______________________________________
Volume of gas collected (mL) ______________________________________
Number of moles of gas collected
(mol) ______________________________________
Molar mass of unknown metal
(g/mol) ______________________________________
Median molar mass of unknown /mole
____________* For Unknown # 7-___________*
Write these down in your lab
manual on the last page of experiment 7 in case this paper is lost.
Calculate the range for the molar
mass of unknown metal: _____________g/mol
List the experimental errors that
might have caused this range: ______________________________
_________________________________________________________________________________
How could the procedure to determine
the molar mass of the unknown be improved?
_________________________________________________________________________________
_________________________________________________________________________________
Please note that this report sheet
will not be graded unless these question are answered on a sheet of paper and
printout of the completed calculation check are stapled to this sheet.
Experiment Eight: Thermochemistry Extra Credit (1-20
pts)
Introduction
Heat is a form of energy possessed by all matter. The
total heat content (or enthalpy) of a sample of matter at any given temperature
is impossible to determine. Only the change in heat content associated
with a change in physical state, a change in temperature, or a chemical
reaction for a given substance can be determined.
Heat is measured in units of calories. A calorie can
be roughly defined as the quantity of heat energy required to raise the
temperature of 1 gram of water by 1 degree Celsius. The amount of heat that is
required to raise the temperature of one gram of any substance by one
degree Celsius is called its specific heat. Liquid water has a specific heat
of 1.00 cal/ºC-g while iron has a specific heat of only 0.11 cal/ºC-g. This
means that it takes approximately one-tenth as much heat to raise the
temperature of 1g of iron by 1ºC as it does to raise the temperature of 1g of
water by 1ºC. It also means that a given amount of heat will raise the
temperature of 1g of iron approximately ten times more than it will raise the
temperature of 1g of water.
When samples of matter at different temperatures are
brought into contact, heat transfers from the sample at the higher temperature
to that at the lower one until the temperatures become equal. Some substances
however transfer heat very poorly. For example, when hot coffee is poured into
a styrofoam cup, heat does not transfer to the styrofoam of the cup easily.
Consequently styrofoam is a good insulator. Coffee stays warm
longer in a styrofoam cup than it would in a metal cup, because a metal is a
relatively good conductor of heat and therefore contributes to the heat loss.
When liquids or solids at different temperatures are
mixed inside of an insulated container like a styrofoam cup, the final
temperature reached for the mixture depends upon the initial temperatures of
the components, the mass of each component, and finally the specific heat of
each component. An insulated container of this sort can be used to construct a
simple calorimeter.
When phase changes occur, for example, the melting
(fusion) of a solid like ice or the boiling (vaporization) of a liquid like
water, a certain constant quantity of heat is required per gram of substance.
Such quantities of heat are called heats of fusion or heats
of vaporization.
Part A of this experiment involves the determination
of the quantity of heat required to melt 1 g of ice. This is defined as the
“heat of fusion of ice”. Weighed quantities of hot water and ice at known
temperatures (hot water about 50ºC and ice at 0ºC) are mixed in 2 nested
styrofoam cups (calorimeter) to do this. The inner cup is covered with a
plastic lid and a thermometer is inserted through a hole in the lid. The cups
are swirled until the ice melts and the final temperature of the contents is
recorded.
Heat is transferred from the hot water to the cold ice
until the ice melts. The cold water produced from the melted ice is then
warmed from 0ºC by heat transferred from the hot water. This system has a
final temperature between 0ºC and the initial temperature of the "hot
water" (assuming sufficient hot water is present to melt all the ice).
This is true because no external heat is added and no internal heat is lost
through the styrofoam insulator. Under these conditions, the heat transferred
in calories is equal to the number of grams of hot water used multiplied by its
specific heat multiplied by the number of degrees it is cooled. This must also
be the amount of heat absorbed by the ice in melting plus the heat
absorbed by the liquid water that is formed. The heat absorbed during melting
is equal the mass of the ice in grams multiplied by the (presently unknown and
to be determined) heat of fusion of water. The heat required to warm the cold
water (from the melted ice) is equal to the mass of the cold water (equal the
mass of ice that melted) multiplied by its specific heat multiplied by the
number of degrees that it is warmed. In summary,
Heat Lost by Hot
Water = Heat Absorbed by Water Initially Present as Ice
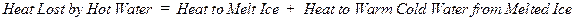
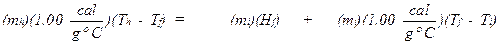
where mh = mass of hot water in grams, mi = mass of ice in grams, Tf = the final temperature that is measured, Th = the initial temperature of the hot
water (in degrees celsius), and Ti =
the temperature of the ice. The heat of fusion (Hf) is the only unknown in the above
equation and it can be solved for as follows:
|
Heat Absorbed by Solid and
Cold Liquid
|
=
|
Heat Lost by Hot Liquid
|
|
Heat to +
Heat to Warm
|
=
|
Heat Lost by Hot Liquid
|
|
Melt Solid Liquid
from Solid
|
|
|
|
Heat to Melt Solid
|
=
|
Heat Lost by Hot Liquid –
Heat to Warm Liquid
|
|
|
|
from Solid
|
|
Heat of Fusion
|
=
|
Heat to melt Solid
|
|
|
|
|
Mass of Solid
|
|
|
|
|
|
|
|
|
|
|
|
For example, determine the heat of
fusion for a metal that has a melting point of 20.0ºC and a specific heat of
0.100 cal/gºC from the following data. If 10.00 g of the solid metal at 20.0ºC
is put in 50.0 g of hot liquid metal at 50.0ºC, the metal melts and the final
temperature of the system is 40.0ºC. Solution:
|
Heat Lost by Hot Liquid
|
=
|
Specific Heat (T)(Mass)
|
|
|
=
|
(0.100 cal/gºC)(50.0 - 40.0ºC)(50.00g)
|
|
|
=
|
50.0 cal
|
|
Heat to Warm Liquid from
Solid
|
=
|
(Specific Heat of
Compound)(T)(Mass)
|
|
|
=
|
(0.100 cal/gºC)(40.0 - 20.0ºC)(10.00g)=20.0
cal
|
|
Heat to Melt Solid Metal
|
=
|
50.0 - 20.0 cal = 30.0 cal
|
|
Heat of fusion
|
=
|
30.0 cal / 10.0 g
|
|
|
=
|
3.00 cal / g
|
Please note that the specific heat for water is
1.00 cal/gºC not the 0.100 list for the metal in this example problem.
Part B of this experiment involves the determination
of the heat of neutralization of a monoprotic acid by a base. The reaction of a
strong acid with a strong base is very exothermic. The amount of energy
released per mole of acid or base neutralized (reacted) is a constant called
the heat of neutralization and has units of cal/mole. When such a reaction is
conducted under insulated conditions, the heat released by the reaction raises
the temperature of the reaction medium. If the mass, temperature change, and
specific heat of the medium is known, the amount of heat produced can be calculated.
You will measure the heat of reaction (called heat of
neutralization) when an unknown strong acid (HX) reacts with a strong base
(sodium hydroxide, NaOH) in a water solution. Since an aqueous strong acid
solution contains H+ (aq) and
an aqueous strong base solution contains OH- (aq), the heat change
will be that due to formation of water.
H+ (aq) + OH- (aq) H2O (l) + heat
Example: 110 mL of 1.00 M NaOH are mixed with 50.0 mL of
2.00 M HCl. Both solutions were initially at 20.1ºC; the final temperature of
the solution (specific heat = 0.960 cal/gºC) after the reaction is 30.3ºC (y
intercept from the graph); thermometer correction factor is 1.020 (see below).
The final mass of the solution is 161.000 g. The energy released by the
reaction and the moles of limiting reagent are needed to calculate the heat of
neutralization.
|
|
Temp.
Change
|
=
|
(30.3-20.1ºC)
|
=
|
10.2 ºC
|
|
|
Corrected
Temp. Change
|
=
|
(10.2 ºC)(1.020)
|
=
|
10.4 ºC
|
|
|
Heat
release
|
=
|
(0.960
cal/gºC)(161.000g) (10.4 ºC)
|
=
|
1607
cal
|
|
|
Moles
NaOH
|
=
|
(0.1100
L)(1.00 mol/L)
|
=
|
0.110
mole NaOH
|
|
|
Moles
HCl
|
=
|
(0.0500
L)(2.00 mol/L)
|
=
|
0.100
mole HCl
|
HCl would
therefore be the limiting reagent (0.110 mol NaOH> 0.100 mol HCl), and the
heat of neutralization would be,
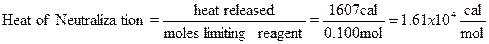
The Experiment
Risk Assessment:
Moderate to High due to corrosives, 2M NaOH and exp. 8 unknown (strong acid).
Thermometer Calibration
Check
Since the measurement of temperature is the greatest
source of error in this experiment, special care needs to be taken to insure
that your thermometer is functioning correctly and that it is read to ±0.1°C.
Checking its calibration will do this. First check and make sure there are no
breaks in the alcohol column inside the thermometer. If there are any breaks
trade the thermometer in for a new one at the lab prep stockroom (room 216). An
easy way to calibrate the thermometer is to compare the temperature difference
measured by your thermometer in ice water (0°C)
to boiling water (93°C at 7,000 feet).
Fill a beaker half full with ice and add a little cold water to make a slush
mixture. First position the thermometer such that the bulb is about in the
center of the water/ice mixture. After 60 seconds measure the temperature.
Gently stir the mixture with the thermometer and recheck the temperature. If
the temperature is constant record it to ±0.1°C on your report sheet, otherwise repeat
this process until the temperature you measure for cold water is constant. If
temperature is not within ±2ºC of zero, check with your TA. Repeat this process
with water heated to a rolling boil in a 400 mL beaker. Take care to keep the
tip of the thermometer in the center of the boiling water. The bulb of the
thermometer should always be place in the center of liquids for good
measurements.
Calculate the difference in temperature between the
ice water and boiling water. This difference should be 93°C. If you determine that the error in
temperature change of your thermometer to be greater than ±4.0°C
from 93°C, label the thermometer with a
piece of tape noting the temperature change. Trade in your thermometer for a
more accurate one at the lab prep stockroom window (room 216).
The measurement of temperature is difficult such that
when the unknown is analyzed a correction factor will be used to make up for
any differences in thermometer calibration. The correction factor (CF) can be
calculated by dividing 93°C by the
temperature difference between boiling water and ice water measured above:
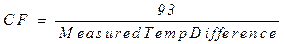
CF will be used to correct the temperature change measured
in determining the heat of neutralization of the unknown, but will not be
needed for the heat of fusion calculations.
Determination of the Heat of Fusion of Water
Obtain 100 mL of hot water from the hot water tap.
Heat the water in a beaker to 50-55ºC if necessary using a hot plate. Weigh
the empty calorimeter (2 clean, dry nested styrofoam cups covered by a plastic
lid available on the side shelf) on the balance. Transfer about 75 mL (using a
beaker) of the hot water (40ºC-55ºC) to the inner cup; place the cover on the
calorimeter and reweigh it to determine the mass of the hot water. Record the
temperature of the hot water to the nearest 0.1C using a thermometer inserted through the hole in the lid.
Add the crushed ice (assume that the temperature of
ice is 0°C, try to avoid adding liquid
water when you add ice) available on the side shelf (ask your TA if none is in
lab), a few pieces at a time and swirl until the temperature is between 15ºC
and 20ºC. Keep the lid on the calorimeter as much as possible during this
period. As soon as the last pieces of ice are gone, read the lowest
temperature reached by the thermometer (to the nearest 0.1ºC) and record it as
the final equilibrium temperature, Tf.
Weigh the calorimeter and contents again to determine the mass of the ice added
and record the data on the Report Sheet. Calculate the heat of fusion for
water using 1.00 cal/gºC for the specific heat of water. Do two trials, and
report the average value. See page 80 for example calculations.
Determining the Molar Heat of Neutralization of an
Unknown Monoprotic Acid (HX)
The unknown for this experiment (8-XXXX) can be check
out from the lab prep stockroom (216). All chemical waste for this experiment
should be place in the liquid corrosives bucket in the hood.
CAUTION: The
unknown is a strong acid (8 M in H+) and is corrosive to skin and
clothing. Handle the unknown carefully and immediately flush any spills with
cold water. The 2 M NaOH is also caustic and should be flushed off
skin immediately. Always wear eye protection in the laboratory. Do not rub
your eyes while working with corrosives. Wash your hands when you are done
working with corrosive chemicals.
Weigh a clean, empty calorimeter on the balance.
Remove the lid and add 50.0 mL of 2.00 M NaOH to the calorimeter
(measured with a 25 or 50 mL graduated cylinder or pump dispenser provided).
Put the lid back on and allow the NaOH in the calorimeter to reach a constant
temperature (this will take 2-5 minutes). Record this temperature to ±0.1º C
on the Report Sheet. Clean and dry the thermometer and measure the temperature
of the acid solution (unknown 8-xxxx). Adjust the temperature of the acid by
heating the unknown bottle in your hand or cooling it in cold water.
When the temperature of the two solutions are within 1C or less of each other, record the temperature of the acid, and clean and dry the
thermometer. Remove the lid, quickly add 10.00 mL of the acid solution into the
calorimeter using a volumetric pipet, start timing, and place the lid back on
the calorimeter. Insert the thermometer and gently swirl the solution for about
20 seconds. Hold the colorimeter securely in your fingertips while swirling,
not with your whole hand, to limit heat transfer from your hand to the
calorimeter. Record the temperature of the solution to the nearest ±0.1ºC at 60 seconds after the addition of
the unknown. Take the temperature every 30 seconds for additional readings at
90, 120, and 150 seconds after adding the unknown. Gently swirl the solution
for about 10 seconds between each temperature measurement.
Remove the thermometer, dry the outside of the
calorimeter with a paper towel, and weigh the calorimeter and
contents (the outside of the calorimeter must be dry before it is
placed on the balance). Dispose of used acids and bases in the “Corrosive
Liquids” container in the hood. The unknown for exp. 8 can be dispose is in
this container also (wait until the it is grade though). Rinse both the
styrofoam cups and used glassware several times with tap water. Make sure
the pipet is also rinsed with distilled water.
Calculations:
Use
the pre-reaction temperature of the 2.00 M NaOH solution as the initial
temperature of the mixture before the reaction starts (since there is about 5
times as much of it). To determine the temperature of the solution after the
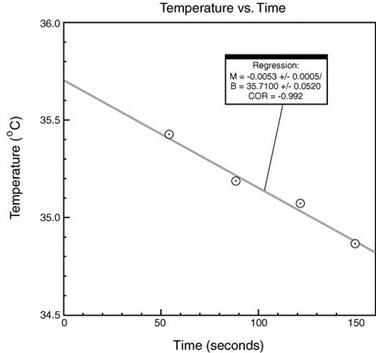
reaction takes place you will first plot the temperatures of the reaction
mixture (y axis) measured at 60, 90, 120, and 150 seconds against the time in
seconds (x axis) using the Graphical Analysis software available in the
chemistry computer labs. Do not plot the temperature of the NaOH.
The y
intercept or temperature at time zero is used as final temperature after the
reaction takes place to compensate for heat lost from the calorimeter to the
surroundings. The y intercept (B) is determined by doing a linear fit. This is
done by selecting the points to be used in the linear fit (select points in
data table with mouse) and selecting the linear fit icon (second icon from
right on tool bar). Make sure the x-axis (time) starts at zero (click on left
hand number on axis and change to zero). Make a graph for each of the two
trials done and print them. If the correlation coefficient (COR) is less than
0.8 see your TA. You may need to discard a point. The y-intercept (B) for the
following example graph is 35.71°C:
The “Change in temperature during the reaction” would
be the y-intercept minus the temperature of the NaOH. This temperature change
can then be corrected for differences in thermometers by multiplying it by the
correction factor (CF) calculated earlier in the experiment. Use 0.960 cal/gC for the specific heat of the contents of the calorimeter (mass of NaOH solution and
unknown acid), the corrected temperature change, and the solution mass to
calculate the “heat released during the reaction”.
Next you must determine whether the OH- in
the NaOH solution (50.0 mL of 2.0 mol/L) or the H+ in the unknown acid (10.00 mL of 8.00 mol/L in H+)
is the limiting reagent by calculating the moles of each. The “Number of moles
of the limiting reagent” is the smaller of the two. Calculate the “Heat of
neutralization” of the unknown acid using the “Heat released during the
reaction” and the “Number of moles of the limiting reagent” (hint - look at the
units). Refer to the limiting reagent example problem earlier in this
experiment and your lecture text for help doing the heat of neutralization
calculations if you still have problems. Clean up your work area with a damp
sponge. Wash the calorimeter and your glassware. Make sure the
volumetric pipet is rinsed several times with tap water and once with distilled
water before storing it.
Use the computer calculation check for this experiment
and attach a copy of it to your report sheet before turning in your results.
Record the following values below and turn in the report sheet at the chemistry
stockroom for grading.
Name__________________________________________ Student
ID#_________________ Dana ID_____
Lab Section Letter__________________ Locker #________________ Date__________________________
Experiment
8 – Thermochemistry Extra Credit
Outline
Experimental Procedure:_________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
____________________________________________________________________________________
Risk Assessment:______________________________________________________________________
TA Signature and Date for Procedure
& Risk Assessment:_______________________________________
Experimental:
Thermometer Calibration Check:
Temperature of ice water (ºC)_________ Temperature of boiling water (ºC)______
Temperature change (ºC)_____________ Correction Factor (93ºC ¸ temperature change)_________
Determination
of the Heat of Fusion of Water: (1) (2)
Mass of styrofoam calorimeter __________________ g
___________________ g
Mass of styrofoam calorimeter + mass hot
water __________________ g ___________________ g
Mass of hot water (mh)
__________________ g ___________________ g
Temperature of hot water (Th)
_________________ ºC __________________ ºC
Temperature of ice (Ti)
_________________ ºC __________________ ºC
Final temperature of water (Tf)
_________________ ºC __________________ ºC
Temperature change of water from ice (Tf-Ti) _________________ ºC __________________ ºC
Temperature change of hot water (Th-Tf) _________________ ºC __________________ ºC
Mass of calorimeter, water, and ice __________________ g
___________________ g
Mass of ice added (mi) __________________ g
___________________ g
Heat lost by the hot water (Hhw) _________________ cal
__________________ cal
Heat used to warm the water from melted
ice (Hiw) _________________ cal __________________ cal
Heat used to melt the ice (Hhw
-Hiw) _________________ cal __________________ cal
Heat of fusion for ice Hf
= (Hhw -Hiw)/ mi ________________ cal/g
________________ cal/g
Average
heat of fusion for ice _____________________ cal/g
Calculate the range for: Heat of
Fusion Water ___________cal/g
Determination of the Molar Heat of Neutralization of an Unknown
Monoprotic Acid:
(1) (2)
Mass of styrofoam calorimeter (g) ________________________________
Temperature of 2.00M NaOH, Initial
Temperature (C) ________________________________
Temperature of unknown 8.00M acid (C) ________________________________
Temperature at 60 seconds (C) ________________________________
Temperature at 90 seconds (C) ________________________________
Temperature at 120 seconds (C) ________________________________
Temperature at 150 seconds (C) ________________________________
Mass of calorimeter, NaOH and unknown
acid (g) ________________________________
Mass of NaOH solution and unknown acid
(g) ________________________________
Temperature from graph extrapolated
back to 0 seconds (C)
(the y intercept used as final temperature of
reaction mixture ________________________________
from plotting temperatures you measured against four times
above as shown in instructions)
Change in temperature during reaction
(C)
(Temperature NaOH or initial temperature – y intercept) ________________________________
Corrected change in temperature during
reaction (C)
(change in temperature during reaction times correction
factor) ________________________________
Heat released during reaction (cal)
(specific heat solution = 0.960 cal/g-ºC, ________________________________
Specific heat x mass NaOH solution and acid x Corrected
Change in Temperature)
Number of moles of the limiting
reagent (mol) ________________________________
(see prelab question number 2 on previous page)
Heat of neutralization (cal/mol) ________________________________
Average heat of neutralization________________________ cal/mol* Unknown
#________________ *
Calculate the range for the Heat of
Neutralization ___________cal/mol
Have you done a calculation check?
(Yes/No)___ If not, do one and staple a copy of it to this report sheet before
submitting your results. Copies of
your graphs must also be attached to this sheet for it to be graded.
*Write these down in your lab manual on the last page of
experiment 8 in case your paper is lost.
Why do we extrapolate back to time zero to determine the final
temperature?
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
What are the major sources of experimental error for this
experiment:__________________________________
______________________________________________________________________________________
______________________________________________________________________________________
![]()
![]()
![]()