EXPERIMENT 3
SEPARATION OF ORGANIC MIXTURES AND IDENTIFICATION
OF ORGANIC COMPOUNDS
INTRODUCTION
A common problem encountered in chemistry involves the separation of a mixture of two or three compounds into single compound fractions followed by the purification and identification of each. To effect the separation, the chemist must make use of the different properties of the components.
We will use differences in solubility, density, acid-base chemistry and reactivity to separate a mixture of compounds. We will then purify and identify each component. The components will be unknown to the student except that one will be a liquid neutral organic compound with a high boiling point and the other a carboxylic acid. The carboxylic acid can react with a base such as sodium hydroxide to form an anion which is water soluble. The neutral will not react and so it will remain “neutral”. The possible organic neutral compounds are listed below:
Table 3-1: Possible Neutral Unknown Compounds and Boiling Points
HYDROCARBONS
Decalin 190°C (2021) o-Xylene 144°C (2213)
![]()
![]()
ALCOHOLS
Cyclohexanol 160°C (2213) Benzyl alcohol 205°C (2213)
![]()
![]()
KETONES
Cyclohexanone 157°C (2213) Acetophenone 200°C (1212)
![]()
![]()
NITRILES
Malononitrile 219°C (2223) Benzonitrile 191°C (1211)
![]()
![]()
Carboxylic acids are compounds which include one or more carboxyl functional groups.

A functional group can be defined as an atom or group of atoms in a definite structural arrangement that influences the properties of an organic compound. The carboxyl group gives the following possible unknowns their acidic character.
TABLE 3-2 POSSIBLE ACID UNKNOWNS
ORGANIC CARBOXYLIC ACIDS
Compound Melting Range (°C) Hazard Code
o-Toluic Acid (2-Methylbenzoic acid) 103-105 1112
Azelaic Acid 106-107 0112
m-Anisic Acid (3-Methoxybenzoic acid) 105-107 1112
2-Phenoxypropionic acid 116-119 3112
Benzoic Acid 121-123 1213
Sebacic Acid 131-134 1113
Cinnamic Acid 132-135 2112
1-Naphthoic Acid 157-160 2112
Salicylic Acid 158-160 2112
p-Toluic Acid (4-Methylbenzoic acid) 180-182 1112
p-Anisic Acid (4-Methoxybenzoic acid) 182-185 1112
Once the unknown neutral and acid compounds have been separated and purified they will then be identified, the neutral compound via its infrared spectrum and the carboxylic acid via its melting range.
Extraction
The general formula for a carboxylic acid is

where R stands for any group of atoms attached to the functional group COOH. As with any Bronsted acid, a carboxylic acid reacts with hydroxide ion, OH-, to produce the conjugate base of the acid and water
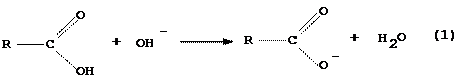
Carboxylic acid Carboxylate anion
insoluble in water soluble in water but
but soluble in insoluble in organic
organic liquids liquids
As is indicated, this reaction also changes the solubility properties of the acid molecule. We will take advantage of these property changes in separating the acid from the mixture.
The neutral component of the mixture may be any one of the hydrocarbons, alcohols, ketones, or nitriles listed in Table 3-1. Neutral compounds will not react with either an acid or a base. They are also water insoluble but very soluble in organic liquids.
The Separation of the Aqueous and Organic Layers
We have now identified the solubility properties of the two components of the mixture which will allow us to separate them. We will use liquid-liquid extraction to take advantage of the differences in solubility of the components. The organic liquid or solvent will be tert-butyl methyl ether (TBME) and the polar aqueous layer will be 5% NaOH or water.
Initially the mixture of the neutral organic and carboxylic acid unknowns will be dissolved in TBME forming an organic solution. Suppose that this organic solution is shaken with a dilute aqueous sodium hydroxide solution and then allowed to stand until the two layers separate. During the shaking process, the hydroxide ion
will
react with only the carboxylic acid ![]() component of the mixture to form
the water soluble carboxylate anion (see Equation 1 again). This changes the
solubility properties of the acid as already stated and results in most of it
moving from the organic liquid layer to hydroxide ion-water layer. The
carboxylate ion thus is the solute and is extracted into the aqueous
(water) phase. The two phases are then separated into two fractions: The
aqueous sodium hydroxide solution containing the carboxylate anion as its salt
and the organic layer containing the neutral organic compound.
component of the mixture to form
the water soluble carboxylate anion (see Equation 1 again). This changes the
solubility properties of the acid as already stated and results in most of it
moving from the organic liquid layer to hydroxide ion-water layer. The
carboxylate ion thus is the solute and is extracted into the aqueous
(water) phase. The two phases are then separated into two fractions: The
aqueous sodium hydroxide solution containing the carboxylate anion as its salt
and the organic layer containing the neutral organic compound.
The two phases will separate and form two separate layers based on differences in polarity and density. The organic layer is much less polar and has a much lower density compared to the diluted NaOH solution. Sometimes the difference in polarity and/or density of the two phases may not be great enough to effect a separation causing the formation of an emulsion. The separation can sometimes be improved by adding more NaOH solution or TBME solvent.
It is important to note that single extractions do not necessarily yield complete separations, and that multiple extractions may be needed. In your work, you will extract the original organic solution two times with aqueous sodium hydroxide solution to remove the acid and water soluble impurities from the organic layer. The two aqueous extracts are then combined and set aside as the aqueous sodium hydroxide fraction. The organic solution is further extracted once with distilled water to remove any water soluble impurities. Once these extractions are complete, the organic solution should contain only the "neutral" compound and the organic acid unknown should be extracted into the NaOH solution.
Purification
Once the two components have been separated, we must obtain each of them in a pure form so they may be easily identified. The pure carboxylic acid in this sequence is a solid while the neutral compound is a liquid. The water soluble carboxylate anion will be precipitated from the sodium hydroxide extract by adding 6M HCl and then recrystallized for purification. The pure neutral compound can be obtained by distilling the lower boiling solvent (TBME) off, leaving only the pure neutral liquid.
Precipitation of the Acid
The carboxylic acid is extracted into an aqueous hydroxide solution because the carboxylate anion RCOO- dissolves more readily in water than in the organic solvent because the anion is SOLVATED by the polar molecules. What would happen if hydrochloric acid, HCl, were added to this carboxylate anion fraction? The hydroxide ion would first react:
OH- + H3O+ ® 2 H2O
and then after the hydroxide ion has been consumed, the carboxylate anion would react to yield the original acid

Since the carboxylic acid has a very limited solubility in water most of it will precipitate; that is, the solid will form in the solution. The solution is then filtered to separate the acid crystals from the aqueous solvent, water. In general, a solid which forms rapidly is not very pure because the crystal lattice includes impurities and must be recrystallized for further purification.
Purification of the Neutral Compound
The mixture of TBME and the neutral compound will next be treated with a drying agent to remove any trace water before the TBME is removed by simple distillation. The pure, neutral compound in this experiment is a liquid which has a characteristic boiling point significantly higher than that of the organic solvent, TBME which has a boiling point of 55°C and density 0.74 g/ml. Simple distillation will then be used to remove the TBME from the neutral compound. The TBME collected will be recycled. A "rotary evaporator" will then be used to remove the final traces of TBME from the neutral organic compound. The rotary evaporator is used to conduct a form of vacuum distillation and can be used whenever the compounds which need to be separated have very different boiling points. The organic solvent evaporates easily when heated at the low pressure, leaving the neutral compound behind.
Identification
The acid in the unknown will be identified by melting point and mixed melting points while the neutral compound will be identified by infrared analysis. Read and study the information in the appendix on these two techniques.
EXPERIMENTAL
At first, it is remarkably easy to get lost in this sequence! The flow chart on the next page is designed to help you get an overview of the procedure to help keep track of where you are and where you are heading. If you do get lost hug the nearest tree until you are found (just kidding).
PRELAB ASSIGNMENT: Review information on acid-base concepts, solvation, extraction, recrystallization, simple distillation, infrared spectroscopy, melting temperature determinations, drying agents in this experiment, the appendix, previous experiments, and the lecture text. Then answer all of the pre-lab questions at the end of this experiment!
Experimental Procedure Flowchart
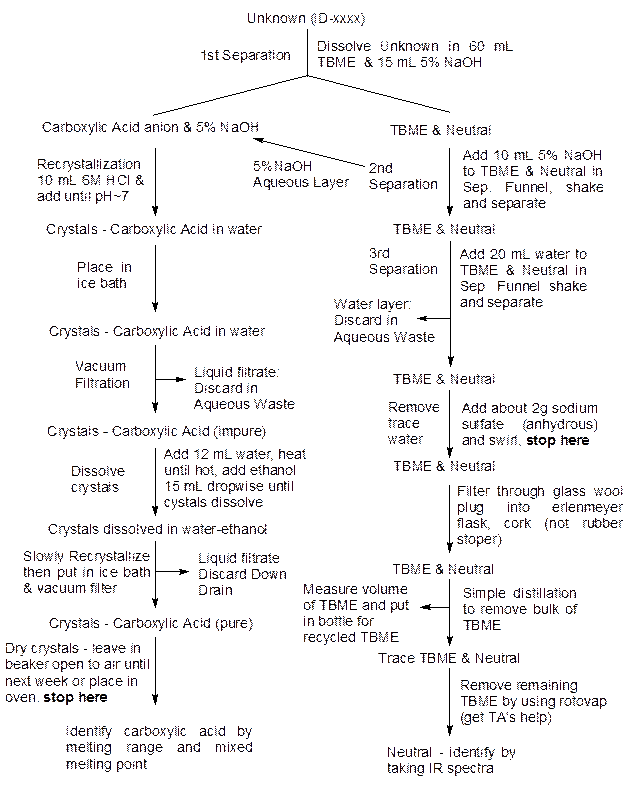
Glassware and Equipment:
Week One: 2-4 100-150mL beaker and erlenmeyer flasks, separatory funnel and glass stopper, Two 125mL erlenmeyer flasks or small depending on need with stoppers (one rubber and one cork), vacuum flask, vacuum tubing, 3-prong clamp, ring stand, buchner funnel with rubber adaptor and fitted filter paper, pH paper, hot plate/magnetic stirrer, and ice bath. The ice machine is located in room 210.
Week Two: glass funnel, glass wool, graduated cylinder, rotovap, infrared spectrophotometer (FT-IR), digimelt, cappillary tubes (closed end).
Simple Distillation: 100 and 250mL round bottom flasks, 3-way connecting tube, West condenser, vacuum connecting tube, straight tube adaptor, two or three ring stands, thermometer, thermometer holder, 250mL heating mantel and voltage controller, 3-4 clamps and clamp holders, ring support, boiling chips, ice.
Chemicals:
60mL tert-butyl methyl ether or TBME (2312), 40 mL RO water, anhydrous sodium sulfate - Na2SO4 (1121), 25mL 5% Sodium Hydroxide – NaOH (2013), ~12mL 6M Hydrochloric Acid -HCl (3024), ~15mL 95% ethanol (0301), ID unknown (3223) that can contain chemical combinations from tables 3-1 and 3-2.
WARNING: Save your purified acid and neutral organic compounds until you have received a grade.
Extractions:
1. Obtain 60 ml of tert-butyl methyl ether (TBME is a non polar solvent and has a B.P. of 55°C and density 0.74 g/ml) from the wall hood placing it in a stoppered Erlenmeyer flask for transport to your hood space. TBME is the organic solvent used in the separation.
WARNING: tert-butyl methyl ether & the unknowns are organic compounds are flammable & may be toxic and irritants. Avoid contact with skin or eyes & inhalation. Do all work in a fume hood. Flush with water in case of contact & notify instructor. Keep these compounds away from open flames. Wear goggles & gloves at all times.
2. Transfer your entire unknown (which is labeled ID-xxxx) into the 125 ml separatory funnel. Use a ring stand from the lab and a ring clamp and wire triangle from your locker to hold the funnel. Use some of your TBME to rinse the unknown from the vial into the funnel. Then add the rest of the TBME to the funnel.
WARNING: 5% NaOH is a strong base. Avoid skin or eye contact. Flush with water for 15 minutes in case of contact and notify your instructor. Wear goggles and gloves at all times for this experiment. The use of lab coat or apron is suggested.
3. Add 15 ml of 5% sodium hydroxide solution to the funnel. Place a lightly greased stopper in the funnel. Turn the funnel upside down being sure to keep the stopper held firmly in place. Grasp the stopcock in the other hand so it will not fall out and shake. Release the internal pressure often by inverting and opening the stopcock momentarily (point the funnel tip into the hood and away from colleagues!) After shaking well, place the funnel right side up in a ring stand and allow the phases to separate into two layers. If the layers do not separate cleanly, obtain the assistant's or instructor's help in "breaking" the emulsion (cloudy layer) formed. Adding more TBME or 5% NaOH and shaking again may help break the emulsion. You want all of your solid acid (ROOH) to be dissolved at this point. If it has not, seek counsel from the TA/instructor then add 5 ml of 5% NaOH, shake vigorously, let settle and repeat until dissolved. Enter the amounts added or changes in procedure in your lab notebook. If the emulsion cannot be broken, put it in the bottle labeled "Aqueous Waste" and start over with a new unknown obtained from the prep stockroom (216).
4. Most of the unknown carboxylic acid (ROOH) has been extracted from the organic (upper layer) phase into the aqueous (bottom layer) phase. We want to save the aqueous extract and extract the organic phase a second time. If you are not sure which layer is organic, consider the densities of the molecules involved. As a last resort, you can always do the "water trick." Simply add a few drops of distilled water to your separatory funnel and observe which layer the water drops add to. The idea is that the water will add to the water layer, like dissolves like. Remove the stopper from the funnel and drain the aqueous solution into an erlenmeyer flask. Try to separate the two phases as sharply as possible. The organic and aqueous layers must separate cleanly. If you have an emulsion between the layers, obtain help from the TA to separate the layer. Be very careful to label the flasks carefully so that you know what is in it, e.g., aqueous 5% NaOH extract with unknown acid or organic solution with neutral unknown.
5. Add about 10 ml of 5% sodium hydroxide to the organic solution and swirl and then shake to extract the solution as before.
6. Drain the aqueous layer and pour it into the flask which contains the first aqueous extract. Stopper the flask or cover it with parafilm and save it for further work.
7. What should be in the organic and aqueous solutions now? Answer this in your notebook. Now extract the organic solution a final time with 20 ml of distilled water to remove any water soluble impurities just as you did before. The distilled water can be discarded in the container for “Aqueous Waste”.
8. The remaining organic solution may be saved in another erlenmeyer flask until you are ready to start your distillation. Add about 2 grams of Na2SO4 to the organic solution. Stopper the flask with a cork (wood). A rubber stopper could absorb the organic solvent, TBME, and swell completely out of normal proportions. Swirl this solution and store it. Record your observations of the organic solution before and after adding the drying agent.
As you read the following steps, you should realize that there will be time to work on more than one of your components during the same lab period. Start to develop the knack of planning ahead to dovetail two or more parts of your experiment.
Purification of the Unknown Acid:
1. Transfer the sodium hydroxide extract to a beaker. Use a little distilled water to make the transfer as quantitative as possible.
WARNING: 6 M HCl is a strong acid. Avoid contact with eyes and skin. Wear safety goggles at all times. Flush well with water in case of contact and notify the TA or instructor.
Let's stop and think about the chemistry at this point:
RCOO-Na+ + HCl ® RCOOH + NaCl
The carboxylate anion (RCOO-Na+) is water soluble. We will add an excess of acid to ensure complete precipitation. RCOOH is not water soluble.
2. It would be to your advantage to review the preliminary discussion on precipitation of the acid. Neutralize the NaOH extract by adding 10 ml of 6 M hydrochloric acid slowly and with stirring in a beaker. When the hydroxide ion is neutralized the unknown acid will begin to precipitate. Keep adding 6 M HCl until the solution is acidic (pH<7, test with pH paper). Then add about 2 ml of excess hydrochloric acid to ensure complete precipitation of the acid.
3. Cool the solution in an ice bath to a temperature of about 5°C because solubility decreases as temperature decreases! Ice is available in room 210. Cool some distilled water at the same time. Collect the newly precipitated acid using vacuum filtration and wash it with the cold distilled water. Use the right size of filter paper and seal it against the inside bottom of the funnel with a little water while the vacuum is on. You may want to save the filtrate (the solution in the flask) until you are sure you have a good yield of crystals, otherwise put it in the container for “Aqueous Waste”. BUT IF YOU DO SAVE THE FILTRATE, DO NOT COMBINE THE IMPURE FILTRATE CRYSTALS WITH THE PURE CRYSTALS REMAINING IN THE FILTER FLASK UNTIL YOU CONSULT THE TA.
4. Use a spatula to scrape the acid from the filter paper into a 150 ml beaker for recrystallization.
Recrystallization
1. Add about 12 ml of distilled water to the beaker and heat the solution on a hot plate so that it just boils. Add 15 ml of 95% ethanol dropwise until any remaining solid dissolves.
WARNING: Ethanol is flammable! Do not use near open flame!
2. If the solid dissolves in about 10 minutes, set the flask aside to let it cool slowly to room temperature, allowing the unknown acid to recrystallize.
3. If the acid does not dissolve completely, add about 5 ml of ethanol dropwise and reheat. Continue adding 5 mL portions of ethanol and heating until the acid does dissolve when the solution is hot. Then allow the flask to slowly cool to near room temperature to obtain purified acid crystals. The solution needs to be cooled slowly since rapid crystal formation will trap impurities in the crystal lattice and defeat the purpose of recrystallization. If you do not have crystals at room temperature check the pH of the solution at room temperature. If the pH is below 7 continue to step #4. If the pH is above 7, add 6M HCl drop wise, until the pH is below 7. There may also be too much solvent some of which will have to be boiled off if crystals do not form.
4. After the crystals have formed and the solution has returned to near room temperature, cool the solution in an ice bath for 10 minutes, recover the crystals by vacuum filtration and wash them with cold distilled water.
5. Scrape the crystals from the filter paper into a pre-weighed beaker to allow them to air dry until the next lab period or oven dry for an hour at about 90°C before determining the melting point. While the crystals are drying proceed to the next part of the experiment. Clean the funnel and filter flask and wash the filtrate down the drain if your yield of acid crystals is good. Reweigh the beaker next week to get the mass of the acid crystals. STOP HERE UNTIL NEXT WEEK.
Removal of water and the solvent, TBME, from the Organic Neutral
It is desirable to purify the liquid, neutral compound before obtaining an infrared spectrum. If your previous separation of the unknown acid was incomplete, the acid might very well contribute to the spectrum and confuse the issue. TBME may also contribute to the spectrum if not completely removed. After removing the TBME by simple distillation and then vacuum distillation (using a rotary evaporator), you should be able to obtain a very pure neutral compound for infrared analysis.
Another possible contaminant is water. The water could be removed by distillation but it is easier to use the following method. The sodium sulfate (solid), which was previously added to the TBME and organic neutral unknown mixture will absorb the water in solution by forming a hydrate. The solution will now be collected using gravity filtration. Support the glass funnel with the ring support and place a plug of glass wool or filter paper in the funnel. Place a clean, dry 250 mL round bottom flask under the funnel and pour the TBME containing your neutral unknown into the funnel slowly. The dry, filtered TBME solution can be stoppered and set aside for the simple distillation. The solid sodium sulfate can be washed down the sink with water after the residual TBME has evaporated. (What additional peak would you see on your infrared spectrum if the water is not removed?) Record the answer in your lab notebook.
Simple Distillation
The bulk of the TBME will be removed from the decanted "dried" TBME solution using a simple distillation procedure. Refer to the Appendix page A-2, figure 1, for a drawing of a simple distillation apparatus and read the information on distillation procedures for more background information. A simple distillation will be used to remove most of the TBME since this organic solvent is an environmental hazard. The pure, distilled TBME that is collected in the receiving flask will be recycled and used again for this experiment.
Assemble the simple distillation apparatus using a 250-mL round bottom flask for the container to be heated and the 100 mL round bottom flask as the receiving flask. Place 2-3 boiling chips in the flask. Use a heating mantle initially as your heat source. Get the TA or instructor to approve your apparatus before beginning. Adjust the heat so that the solution comes to a boil but never boils over the top of the flask. Monitor the temperature as the distillation proceeds and record any temperature changes. Continue the distillation until the temperature starts dropping. Then remove the heat and allow the flask to cool.
Remove the receiving flask containing the pure distilled TBME, measure the volume of distilled solvent, and pour it in the bottle in the hood labeled "Distilled TBME". Do not put anything else in this bottle! Now a rotary evaporator will be used to remove residual TBME from your unknown neutral compound. Your assistant or instructor will then help you to remove the remaining residual TBME using a rotary evaporator. The liquid remaining should be the neutral compound. Measure the volume of this liquid in mL. Calculate % of TBME recovered based on the volume collected in the distillation and the volume used in the extraction.
Identification of the Neutral Unknown by Infrared Analysis
Obtain an infrared spectrum of your pure, liquid neutral compound. Use this spectrum to determine the functional groups that your neutral unknown does and does not have and then identify it. Save your organic neutral until your graded unknown report sheet is returned to you. It can then be discarded in the bottle labeled "Acetone Waste".
Identification of the Unknown Carboxylic Acid by Melting Point
Measure the mass of unknown collected and then measure the melting point. You may find it possible to save time by preparing two capillary tubes--using the first tube to find an approximate melting range with rapid heating and the second tube to find a more accurate melting range with slower heating. Remember to always report a melting range with the initial temperature at which liquid is observed and the final temperature at which the solid disappears.
You will then have to do a mixed melting point to determine its identity as was done in the melting point experiment. Save the purified unknown carboxylic acid until you are satisfied with your grade and then place it in the waste bottle marked "Waste Organic Solids".
Experiment 3 Study Guide – Prelab Questions
A quiz will be given from the prelab assignment, the prelab lecture, and this study guide before the start of the experiment.
1. The presence or absence of key functional groups will enable you to identify the neutral organic compound in your unknown. List these key functional groups (hint: see table 3-1):
2. If you were given a separatory funnel and told it contains an organic and aqueous layer, how could you determine which layer was aqueous?
3. Express the density of the solvents in units of grams/mL. Which is denser: TBME or H2O? Which would be the top layer?
4. What component of the unknown solution (neutral or acid), is extracted into the organic solvent and which is extracted into the aqueous 5% sodium hydroxide?
5. Explain the basic chemical principles by which you will separate your organic acid from the neutral compound. Use chemical reactions and solvent polarity to help answer this question.
6. Explain how the carboxylate anion (RCOO-) is converted back to the unknown acid and recovered. (use a chemical reaction in your explanation)
7. What is the purpose of the recrystallization step? Why wash the crystals with cold water instead of room temperature water?
8. How are the TBME and the H2O removed from the neutral unknown to purify it? What is the purpose of sodium sulfate in this experiment, and how much do you add?
9. If the melting range is 104-106°C what acid from table 3-2 is it?
10. Three liquids have the densities noted and are not soluble in each other: A - 1.01 g/mL, B - 1.31 g/mL, C - 0.72 g/mL. If equal volumes of the three liquids are mixed together which will be on the top, middle and bottom? Why?
11. The IR spectrum for an unknown has a strong doublet peak at a wave number of about 2950 cm-1 and no other peaks until 1500 cm-1 or less. Which neutral unknown listed on page 3-1 is it? Why?
12. Risk Assessment: What are the safety hazards and precautions for this experiment?
ORGANIC SEPARATIONS - EXPERIMENT 3
CHM 235L REPORT SHEET FOR UNKNOWN ID-XXXX
Student’s Name ____________________________ Dana ID_________ Unknown #________
Section Letter__________ Locker #__________________ Work Station #_______________
Teaching Assistant_________________________ Instructor_________________________
Turn in this report sheet to the box inside the prep stockroom window (room 216).
Do not put this sheet in the notebook. Do not write the hazard code, which has the form HC-xxxx, for the unknown number. The unknown number can be found on the vial containing the unknown or, if the vial is missing, the unknown number should also be recorded in the blue book in the prep stockroom. The unknown number on the vial starts with ID-.
UNKNOWN ACID:
Melting Range oC (from slow run) _________________________ Mass (g)_______
50:50 Mixtures of Know Possible Compounds With Unknown
Compound Name Melting Range ( oC)
______________________ ________________
______________________ ________________
Identity of the Organic Acid Unknown_____________________________________
UNKNOWN NEUTRAL:
List All Functional Groups, Wave Number, Present___________________________
____________________________________________________________________
____________________________________________________________________
Identity of the Organic Neutral Unknown _______________________ Volume (mL)_______
Percent Yield or Recovery of TBME________________________
Teaching Assistant's Initials For Checking This Sheet__________
NOTE: This does not guarantee you identified your unknowns correctly!