Experiment 5: Alcohol Dehydration of Menthol
OBJECTIVE: Synthesize menthene from menthol via an alcohol dehydration reaction. Product identity and purity are evaluated using boiling point (BP), infrared spectroscopy (IR), and gas chromatography (GC).
INTRODUCTION: Acid catalyzed, dehydration of a secondary or tertiary alcohol is an E1 elimination reaction, accomplished by the addition of a strong, concentrated mineral acid and high temperature; the absence of the acid catalyst inhibits product formation. The temperature and acid concentration required for dehydration are dependent on the alcohol structure. The relative ease with which alcohols undergo dehydration is in the order: 3o > 2o > 1o. The traditional laboratory experiment has been the dehydration of cyclohexanol to form cyclohexene (below) which is inexpensive to conduct and occurs in high yield.
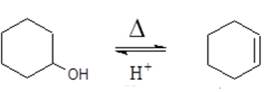
In an effort to make this experiment green, menthol was chosen as an alternative reagent to cyclohexanol. Commercially available menthol exists as an inexpensive racemate and its use as a reagent in lieu of cyclohexanol reduces the overall NFPA health and flammability ratings of this reaction making it an acceptable bench top experiment, eliminating the need for a chemical fume hood. Both cyclohexanol and cyclohexene are flammable liquids; cyclohexene is a suspect carcinogen so care must be taken to contain the product’s fumes which can become overwhelming, an issue that is eliminated with menthol as the reagent.
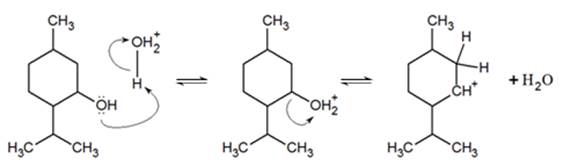
In this reaction, the hydroxyl group is protonated by the acid catalyst where subsequent dissociation of water gives an unstable, carbocation intermediate.
Once the carbocation is formed, water will attack a proton of an adjacent carbon forming a carbon-carbon double bond and regenerating the acid catalyst. The double bond can form between either carbon adjacent to the carbocation, forming two distinct products: 1-isopropyl-4-methyl-cyclohex-1-ene (1-menthene) and 1-isopropyl-4-methyl-cyclohex-2-ene (2-menthene). Zaitzev’s rule tells us the major product will be 1-menthene.
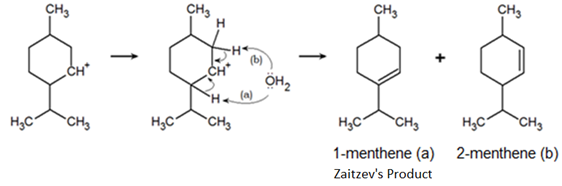
Zaitzev’s Rule states that elimination is favored to give the most stable, most highly substituted alkene. Here the carbon-carbon double bond is formed between the neighboring tertiary carbon in excess of 90% when contrasted with the neighboring, secondary carbon.
Upon formation of the carbocation, rearrangement called a hydride shift can occur through the migration of a proton, with its electrons, from an adjacent carbon to the carbocation. During the transition state, the shifting hydride is partially bonded to both carbon atoms by the electron pair with which it migrates and never fully leaves the ring.
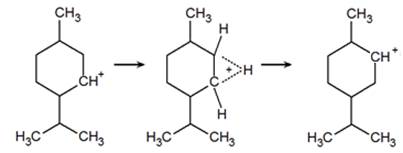
The hydride shift repositions the carbocation allowing the formation of a third product. Just as had occurred previously, the formation of two unique products is possible. A carbon-carbon double bond can form 2-menthene (c) however, as seen before, preference for the more stable, tertiary carbocation, forming 1-methyl-4-isopropyl-cyclohex-1-ene or 3-menthene (d). As a result, the alcohol dehydration of menthol produces three similar, yet differing products in varying quantities.
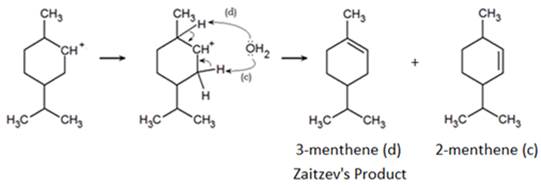
Since menthene and menthol exist in equilibrium, it is difficult to convert all the menthol to menthene. Here we employ Le Chatelier's principle which states that the equilibrium between reactants and products shifts to favor product formation if heat is added or a product is removed.
After reflux, menthene is isolated from the reaction flask by fractional distillation. After the distillation, the product will have trace contamination. Product workup is done by neutralizing excess acid with sodium bicarbonate and washing with water. The isolated product is then dried over sodium sulfate and in week 2, simple distillation further isolates the product from any remaining menthol. Product purity and composition will be determined by infrared spectroscopy and gas chromatography after the second distillation.
EXPERIMENTAL PROCEDURE:
Prelab Preparation:
· Preparation of alkene via alcohol dehydration (lecture text).
· Fractional and Simple distillation (appendix).
· Gas chromatography and infrared spectroscopy (appendix).
|
Chemical |
Boiling Point (oC) |
Flash Point (oC) |
Health |
Flammability |
Reactivity |
||
|
Cyclohexanol |
161 |
68 |
2 |
2 |
0 |
||
|
Cyclohexene |
83 |
-12 |
2 |
3 |
0 |
||
|
Menthol |
212 |
93 |
2 |
1 |
0 |
||
|
3-Menthene |
176 |
35 |
0 |
2 |
0 |
||
|
2-Menthene |
165 |
48 |
0 |
2 |
0 |
||
|
1-Menthene |
174 |
43 |
0 |
2 |
0 |
||
|
85% H3PO4 |
158 |
- |
3 |
0 |
0 |
||
CAUTION: Phosphoric acid (2023) is corrosive to tissue. Wash immediately upon contact. Menthol and menthenes are flammable so take care to contain any fumes. Make sure all joints on the distillation apparatus are sealed or you will lose product. Also, be sure to rinse all glassware that contained menthene with acetone in the prep hoods before washing in sink.
Week 1: Reaction and Isolation
Glassware and Materials:
Fractional Distillation: 250mL and 50 mL round bottom flasks, 3-way connecting tube, Liebig condenser, West condenser, water connecting tube, straight tube adaptor, thermometer, thermometer holder, two or three ring stands, heating mantel, 2-3 clamps and clamp holders, ring support, boiling chips or magnetic stir bar, ice water bath, recirculating water pump.
Other Equipment: Separatory funnel with glass stopper, pH paper and waste beaker.
Procedure: Week 1
Set up a Fractional Distillation: Set up the fractional distillation as shown in figure 3 on page A-1 of the appendix. Lightly grease and seal the glassware joints. Use a recirculating pump and small bucket containing ice water.
Mix reactants: Add 25 mL (record to the nearest 0.01 mL) of menthol, 5 mL of 85% phosphoric acid, and about 4-8 boiling chips to a clean, dry 250 mL round bottom flask. Reconnect the 250 mL round bottom flask to the vertical column, start circulating water through the horizontal column and get the TA or instructor to approve your setup before beginning to heat.
Begin fractional distillation: Initially set the heating control at 6 and increase as needed to gently boil the reaction mixture so that the vapors ascend slowly up the Liebig condenser and begin to condense in the collection flask at a rate of about 1 to 2 drops per second. Adjust the controller if the rate of distillation is too fast or too slow. Observe and record the temperature of the first drop of distillate, the temperature range over which the bulk of the distillate is collected, and the temperature at which the heat source is removed. Remove the heat source when you observe white vapors and foam in the distilling flask - this may occur when most of the starting volume has reacted and distilled. The white vapor may be phosphoric acid. Remove the collection flask and keep a stopper on the flask containing the menthene at all times to avoid loss due to evaporation.
Workup: Neutralize the distillate which contains some phosphoric acid, which unavoidably distills with the products, by transferring the distillate to a clean separatory funnel and add 25 mL of 5% NaHCO3 solution. Cap and swirl the solution slowly at first and then shake vigorously, pointed away, to neutralize the phosphoric acid. Allow the layers to separate, drain the aqueous (lower) layer into an Erlenmeyer flask designated as aqueous waste; test the pH of the aqueous layer as it drains from the uncapped seperatory funnel. Repeat the neutralization if the pH is below 5, or continue to the next step if the pH is 5 or greater. Rinse twice with 25 mL portions of water, draining the water into the aqueous waste flask. Transfer the waste container into the labeled “Aqueous Waste” in the fume hood while saving the top layer.
WARNING: THE ADDITION OF 5% SODIUM BICARBONATE TO THE ORGANIC DISTILLATE MAY EVOLVE A LARGE AMOUNT OF CARBON DIOXIDE GAS. ADD IT SLOWLY AND SWIRL THE SOLUTION AS THE NaHCO3 IS ADDED.
Drain the top organic layer, into a clean and dry 25-50 mL E. flask (weigh with stopper). Expect a trace amount water to transfer with the sample. Reweigh the flask with the product to get the mass of menthene that you synthesized. Add approximately 0.1 g of anhydrous sodium sulfate per milliliter of product obtained, swirl, and stopper with rubber stopper until the next lab period. This will remove water from the product. If time does not allow for instrumental analysis, label the flask with your name and section letter and give to your TA to store under refrigeration.
Week 2: Purification and Analysis
Glassware and Materials:
Simple Distillation: 250mL and 50 mL (preweighed with stopper for product) round bottom flasks, 3-way connecting tube, West condenser, water connecting tube, straight tube adaptor, thermometer, thermometer holder, two or three ring stands, heating mantel, 2-3 clamps and clamp holders, ring support, boiling chips or magnetic stir bar, ice water bath, recirculating water pump.
Other Equipment: gas chromatograph (GC) and infrared spectrometer (IR).
Procedure: Week 2
Begin Simple Distillation: Initially set the heating control at 6 and increase as needed to gently boil the reaction mixture so that the vapors ascend slowly up the 3-way connector and begin to condense in the collection flask at a rate of about 1 to 2 drops per second. Adjust the controller if the rate of distillation is too fast or too slow. Observe and record the temperature of the first drop of distillate, the temperature range over which the bulk of the distillate is collected, and the temperature at which the heat source is removed. Do not distill to dryness. Remove the collection flask and keep a stopper on the flask containing the menthene at all times to avoid loss due to evaporation. Add a small amount of sodium sulfate to remove trace water.
Instrumental Analysis: Obtain an infrared spectrum (IR) for the distilled and dried product. Load the salt plates with a Pasteur pipet and run the IR and print your spectrum. The IR of your purified product must be run and shown to your TA before they can run a gas chromatogram (GC). Finally have the TA run your purified product on a GC. Your TA will measure the volume of your cyclohexene and return your flask to you.
Grade Requirements: Your grade is based on product purity and yield. Thoroughly evaluate your IR spectra and gas chromatograph, as well as all of your boiling points in your lab notebook. Determine the theoretical and percent yield of menthene as well as percent purity from your GC and clearly explain your findings and any possible error in the lab notebook.