SAFETY IN THE CHEMISTRY LABORATORY
TABLE OF CONTENTS
SAFETY IN THE CHEMISTRY LABORATORY I-8
Chemical Labels and Hazard Codes I-9
Material Safety Data Sheets and Safety References I-11
Fume Hoods Use and Cleaning Glassware I-13
Fire Safety I-13
How to Protect Yourself I-15
What to Do in Case of Accident I-16
Hazardous Waste Disposal and Handling Reagents I-17
Laboratory safety involves the prevention of and response to laboratory emergencies. Good prevention is far better than someone getting hurt. This begins with always being aware of chemical and laboratory hazards. Hazard codes, chemical labels, and material safety data sheets are the first sources of information that help us prepare to work safely in a laboratory. This information can be used to do risk assessment on an experiment you are about to do. Certain rules need to be followed to keep you safe and you must know what to do in case of an emergency. Chemical waste management is another important aspect of a safe laboratory and a key regulatory compliance issue.
Chemical Labels and Hazard Codes
The first source of information is the label on a chemical bottle. Read the label carefully before using a chemical. A commercial chemical bottle will have extensive information on the label such as the chemical name and formula, physical properties, purity, molar mass, hazards, safety precautions, suggested protective equipment, and other information. A hazard code may also be included on the label.
The chemistry department has adopted the “Baker” hazard code classification system to inform users of potentially hazardous chemicals. This system is designed to provide information to people who handle chemicals in laboratories. Hazards are classified according to four types: health (toxic), flammability (fire), reactivity (explosive or reactive), and contact (corrosive). The intensity of the hazard is indicated by using a number from "0" (no hazard) to "4" (extreme hazard). This information is conveyed using either a four-colored label found on "J.T.Baker" chemical products or as a series of four digits. The label on chemical bottles may look like this:

The four-digit hazard code used in the lab manual would look like this:
1321
For example, the code listed above for acetone indicates a slight health hazard (1), a high flammability hazard (3), a moderate reactivity hazard (2), and a slight contact hazard (1). Hazard codes will be listed after the chemical inside parentheses: (1321)
The "Baker Codes" for each of the four hazards are defined according to the following scheme:
HEALTH (BLUE): Toxic effects of a substance if inhaled, ingested or absorbed.
0. No Hazard
1. Slight hazard
2. Moderate hazard
3. Severe danger
4. Deadly, Life Threatening
FLAMMABILITY (RED): Tendency of a substance to burn based on flash point or the temperature a substance will burn when exposed to a spark.
0. Will not burn
1. Flash point above 200°F
2. Flash point from 100-200°F
3. Flash point from 73-100°F
4. Flash point below 73°F
REACTIVITY (YELLOW): Potential of a substance to react violently with air, water or other substances.
0. Stable.
1. Reacts under elevated temperature or when in
contact with other substances under other than normal working conditions.
2. Reacts violently but will probably not explode
under normal working conditions.
3. Reacts violently or explodes under normal working
conditions when in contact with air, water or other substances.
4. May react violently or detonate spontaneously under
normal working conditions.
CONTACT (WHITE): The danger a substance presents when it comes in contact with skin, eyes or mucous membranes.
0. No contact hazard to normal, healthy tissues.
1. Slight hazard; irritant to sensitive tissues,
Avoid contact with eyes and mucous membranes.
2. Moderate hazard; irritant to sensitive tissues,
damages tissues.
3. Severe danger; destroys tissues, including skin.
4. Extreme danger; life threatening.
The National Fire Protection Association (NFPA) is a hazard code system that was adopted in 1975 to communicate hazards to emergency responders. This system uses a label that you may be familiar with since it appears on entrances to stores containing hazardous chemicals and on chemical containers. The NFPA may differ from the “Baker” code since it provides information to firefighters while the “Baker” code provides hazard information in a laboratory situation. The codes are very similar except the white section in the NFPA code refers to special or specific hazards of importance to firefighters such as “ox” for oxidizing agent.
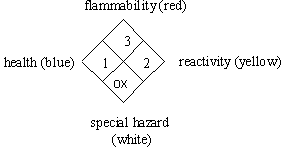
Health Hazard (Blue) - Degree of hazard for short term protection
0. Ordinary combustible hazards in a fire
1. Slightly hazardous
2. Hazardous
3. Extreme danger
4. Deadly
Flammability (Red) - Susceptibility to burning
0. Will not burn
1. Will ignite if preheated
2. Will ignite if moderately heated
3. Will ignite at most ambient conditions
4. Burns readily at ambient conditions
Reactivity, Instability (Yellow) - Energy released if burned,
decomposed, or mixed
0. Stable and not reactive with water
1. Unstable if heated
2. Violent chemical change
3. Shock and heat may detonate
4. May detonate
Special Hazard (White position on diamond)
OX. Oxidizer
W. Use no water, reacts!
The hazard codes are given only as a guide to warn the user of probable hazards and to approximate the degree of hazard under normal use. The user must not be lulled into a false sense of security by a low number on the label, but must take full responsibility for safe use of the chemicals. Avoid over-reliance on hazard codes. Refer to the Material Safety Data Sheets (MSDS) and other safety information whenever you are working with chemicals that are unfamiliar to you. This is especially important when mixing chemicals. Chemicals with relatively safe hazard codes can become dangerous when mixed with other chemicals.
The Material Safety Data Sheet (MSDS) should be read to obtain additional safety information before the chemical is used. These sheets are available in Room 212 for all chemicals used in the chemistry department; they must not be removed from that room. The internet is a great resource for MSDS and general safety information.
MATERIAL SAFETY DATA SHEETS AND SAFETY REFERENCES
Often the label on a chemical container does not provide enough information on a chemical to use it safely. Additional facts on chemical hazards can be found in material safety data sheets, safety references, and chemical/safety catalogs. A safety library is maintained in room 212. Material Safety Data Sheet (MSDS) for every chemical purchased by the chemistry department are maintained and use of these sheets is encouraged. Aldrich/Sigma MSDS volumes are also available. Links to MSDS information can be found on the web under course information on chemistry instructional labs homepage. Other references on laboratory safety, hazardous chemicals, carcinogens, chemical first aid, exposure limits, hazardous waste disposal, etc. are also available. The following references can be found in room 212.
a) CRC Handbook of Laboratory Safety
b) "Dangerous Properties of Industrial Materials" by Sax
c) Toxic Substances List (NIOSH)
d) Annual Reports on Carcinogens
e) Emergency Response Guide
f) "First Aid Manual for Chemical Accidents" by Lefevre
g) The NAU Chemical Hygiene Plan
h) "Safety in Working with Chemicals" by Green and Turk
i) NIOSH/OSHA Pocket Guide to Chemical Hazards
Free sources of information such as chemical catalogs (the Aldrich and Flinn catalog are good references) and safety catalogs can provide information such as the right choice of gloves for a certain class of hazardous compounds. Room 212 is open Monday through Friday from 8:00 am to 5:00 p.m. to make this safety material more available. This material will be removed from 212 for making copies and then only for 30 minutes or less.
The Material Safety Data Sheet (MSDS) provides technical, chemical, physical, and hazard information for the "hazardous material." The "Hazardous Material" may be an individual substance or a mixture of hazardous ingredients. The Occupational Safety and Health Administration (OSHA) requires manufacturers to prepare an MSDS for each hazardous substance they make. While OSHA is very specific about the information that must be provided in an MSDS, they do not require manufacturers to provide the information in a certain format. Therefore, the order in which information is provided in a MSDS may vary from manufacturer to manufacturer. The following is an outline of the content of a MSDS, but not necessarily in the order provided by all manufacturers:
A. Material Identification and Hazardous Components
B. Physical/Chemical Characteristics - vapor pressure, flash point, etc.
C. Fire and Explosion Hazard Data - auto ignition temperature, extinguishing media, etc
D. Reactivity Hazard Data
E. Health Hazard Data
F. Control and Protective Measures - the type of personal protective equipment and type of ventilation to be used, and the precautions to be taken when using the material for its intended purpose is given
G. Precautions for Safe Handling and Storing - use/leak procedures
Please note that more sections and more information may be provided by the individual manufacturer; however, the information required in the listed sections MUST be found in a MSDS. While all of the information in the MSDS is important, the information on the information on safe handling, control and protective measures, reactivity/health hazards, and extinguishing media is very important. When using a MSDS keep in mind that the target audience is assumed to be a chemical worker using larger amounts of the material than you will encounter. The personal protection measures may sometimes need to be modified for a laboratory situation where much small amounts are used in a more controlled environment. For the safety of yourself and those people you work with, be sure to read the MSDS on any chemical you work with. BE INFORMED! Read labels and use care when using concentrated reagents.
FUME HOODS
The building is equipped with state-of-the-art low flow fume hoods. You need to know how use them correctly. First of all, every hood is equipped with a flow sensor and alarm. If the flow is too slow or too fast they may not be able to trap and remove fumes and an alarm will go off. Notify your TA or instructor immediately if this is the case. The fume hood has two modes, standby or sleep mode and normal mode. When the sash (front window of the hood) is all the way closed the hood will be in standby mode and the flow rate will be about half of normal mode to conserve energy. If you pull the sash up about an inch or less the hood flow will ramp up to normal mode and provide you with the best protection. The sash has sliding windows that can be used to protect you and provide access to work inside the hood with the sash closed. The hood may not function correctly with the sash all the way up. Also never store chemicals or equipment in a fume hood because this can degrade fume hood performance. Keeping this information in mind follow these rules for safe fume hood use:
· Never put you head inside a fume hood.
· Keep the sash down except when setting up an apparatus.
· Before using pull sash up just enough to ramp up to normal mode (<1”).
· Use sliding windows to access work in hood.
· Notify the TA or instructor immediately if an alarm comes on.
· Clean up any spills immediately. Get the TA’s help if the spill may be dangerous.
· When done working in a hood, wipe down with a damp sponge, push the sash all the way down, close the sash windows, and turn off the light.
· Never store chemicals and equipment in the hood. Instead use your bin or locker for your solutions or for other stuff the storage cabinets under or to the side of the hood. It’s ok to leave ring stands and rubber tubing for condensers in hood.
CLEANING GLASSWARE
First of all use proper gloves to protect your hands if needed. To properly clean glassware you need to be aware of the hazards and solubility properties of the material that was used. Be sure to properly dispose of any used chemicals if they pose a hazard. You may need to dispose of any residual material in a container if it poses a significant hazard. Containers used for volatile concentrated acids such as hydrochloric, nitric, or acetic acids or bases such as concentrated ammonium hydroxide must be rinsed in a fume hood with water to remove any residual chemical.
Water soluble materials such as ones used in general chemistry labs can often just be rinsed with hot tap water several times and then once with RO water (purified water). Fill a wash bottle with RO to rinse glassware or use the RO rinse tub in you lab. Do not use the RO water tap in you lab to rinse your glassware. Wash glassware soon after you are done using it and never leave or store dirty glassware. If need be use lab soap to help clean glassware. Hard to clean glassware such as volumetric or mohr pipets may need to be cleaned with soap in a sonicator. Sometimes with less water soluble materials you will need to use a cleaner such as simple green. This works very well with labels.
When working with volatile organic chemicals, containers may need to be rinsed with acetone in a fume hood to remove the organic material before washing at a sink. Our organic chemistry labs will have at least one acetone cleaning station in a fume hood. Never allow fumes from a volatile chemical to escape into the lab. Some organic material such as vacuum grease is insoluble in both acetone and water. Use hexanes to remove this material and then acetone to remove the residue hexanes. Collect used acetone and hexanes in a waste bottle. Do not add any other materials to these bottles.
FIRE SAFETY
Using extinguishers:
In most chemistry laboratories there is a fire extinguisher, eyewash and safety shower, first aid kit, and other safety equipment. To use an extinguisher:
a. Select proper extinguisher for fire (explained later)
b. Twist pin and pull
c. Hold the extinguisher in upright position and aim the
hose or bell at base of the flames from a distance
d. Squeeze handle until extinguishing material is released
e. Slowly approach fire sweeping the base of the flames
f. Continue until fire is out
g. Continue to watch for auto ignition even after fire appears out
h. In summary remember "PASS"; Pull, Aim, Squeeze, Sweep
Three Components of Fire:
Fire burns because three components are present- heat, fuel and oxygen. Fire is a chemical reaction. It happens when a material unites with oxygen so rapidly that it produces flame. Think of fire as a triangle. If any one of the three sides, heat, fuel or oxygen is taken away, the fire goes out. This is the basis for fire extinguishment. Heat can be taken away by cooling, oxygen can be taken away by excluding air, fuel can be removed to a place where there is no flame, or a chemical reaction can be stopped by inhibiting the oxidation of the fuel.
Cooling a fire calls for the application of something, which absorbs heat. Although there are others, water is the most common cooling agent. Water is commonly applied in the form of a solid stream, finely divided spray or incorporated in foam.
Often, taking the fuel away from a fire is difficult and dangerous, but there are exceptions. Flammable liquid storage tanks can be arranged so their contents can be pumped to an isolated empty tank in case of fire. When flammable gases catch fire as they are flowing from a pipe, the fire will go out if the flow can be valved off.
Oxygen can be taken away from a fire by covering it with a wet blanket, throwing dirt on it or covering it with chemical or mechanical foam. Other gases which are heavier than air, such as carbon dioxide, or a vaporizing liquid can be used to blanket the fire, preventing the oxygen from getting to the fire.
Studies made during recent years have indicated that the familiar statement, "Remove heat, remove fuel or remove oxygen, to extinguish a fire" does not apply when dry chemical or halogenated hydrocarbons are used as the extinguishing agents. These agents inactivate intermediate products of the flame reaction resulting in a reduction of the combustion rate [the rate of heat evolution] and extinguishing of the fire.
Four Classes of Fires:
CLASS ["A"] fires occur in ordinary combustible materials such as wood, cloth and paper. The most commonly used extinguishing agent is water, which cools and quenches. Special dry chemicals also extinguish fires in these materials for use on Class A, B and C fires. These provide a rapid knock down of flame and form a fire retardant coating, which prevents flash.
CLASS ["B"] fires occur in the vapor-air mixture over the surface of flammable liquids such as grease, gasoline and lubricating oils. Flammable liquids always generate vapor due to their high vapor pressure. When mixed with air and contacted by an ignition source, it is the vapor, not the liquid, which burns. The fuel vapor and oxygen provide two sides of the fire triangle. A flammable liquid is usually more dangerous when temperatures are high because more vapors are generated. The lowest temperature at which a liquid still has enough vapor pressure to give off enough vapors to form a flammable mixture with air is called the flash point.
A smothering or combustion inhibition effect is necessary to extinguish Class "B" fires. Dry chemical, foam, vaporizing liquids, and carbon dioxide all can be used as extinguishing agents depending on the circumstances of the fire.
CLASS ["C"] fires occur in electrical equipment where non-conducting extinguishing agents must be used. Dry chemical, carbon dioxide, and vaporizing liquids are suitable. Because foam, water (except as a spray), and water-type extinguishing agents conduct electricity, their use can kill or injure the person operating the extinguisher, and severe damage to electrical equipment can result.
CLASS ["D"] fires occur in combustible metals such as sodium, potassium, lithium, magnesium, titanium, or zirconium. Specialized techniques, extinguishing agents and extinguishing equipment have been developed to control and extinguish fires of this type. Normal extinguishing agents generally should not be used on metal fires as there is danger in most cases of increasing the intensity of the fire because of a chemical reaction between some extinguishing agents and the burning metal. Since the Chemistry Department doesn't have a Class "D" fire extinguisher, dry sand or graphite can be used to smother Class "D" fires. Buckets of dry sand should be provided in labs where Class "D" fire hazards are present.
How to Protect Yourself
1. Eye Protection MUST BE WORN IN THE LABORATORY AT ALL TIMES unless otherwise notified by the instructor or TA. Avoid rubbing your eyes in lab unless you wash your hands first. Use extra caution when using corrosive chemicals. Indirectly vented or nonvented goggles are the required eye protection for this lab course. Safety glasses or directly vented goggles are not acceptable. Do not modify or remove the vents on goggles.
2. Skin protection should be employed where appropriate; you may be required to wear long pants. Avoid wearing shorts. The use of a lab coat or plastic apron is recommended. Closed toed shoes must be worn at all times in the laboratory for protection against broken glass and spilled chemicals. Open-toed shoes or sandals are not appropriate footwear in lab areas. Disposable gloves are available for the handling of hazardous chemicals. After completing lab work for the day, wipe down your work area with a damp sponge to clean up any spilled chemicals. Rinse and wring out the sponge and then wash your hands.
3. Protection from fumes or fine powders: Never allow hazardous chemical fumes or dust to escape into the open room; use fume hoods when necessary or specified. Be sure to use the fume hoods correctly, following the instructions provided by your TA or instructor. Avoid putting your head inside the fume hood and close the sash or fume hood window when it is not in use.
4. Protection from internal poisoning: Never "pipet by mouth", eat, drink, or smoke in the laboratory. These activities are prohibited. Wash your hands after you have completed lab work.
5 Protection from hot surfaces: Use the appropriate types of tongs to handle hot objects. Test tube holders are too weak for carrying flasks.
6. Protection from fire and explosion: Never allow flammable vapors to escape into the open room (see No. 3). Ether is especially dangerous in this respect. Never use an open flame while flammable liquids are being used in the room. Hot plates should be used with care, as they are an ignition source. Flammable volatile liquids should be used in fume hoods and stored in solvent cabinets when possible. Long hair should be tied back to keep it away from open flames.
7. Protection from cuts: When manipulating glassware or ceramic ware, protect your hands with a cloth towel. Clean up broken glass immediately. Do not pick up broken glass with bare hands. Use a broom and dustpan to dispose of glass in the "Broken Glass Container". Do not clean up broken mercury thermometers without help from your TA since mercury requires special disposal procedures.
8. Protection from the unexpected: Always read all labels noting the chemical name, formula, concentration, and warnings (including hazard codes) carefully and double check to make sure you have the correct chemical and concentration. Follow directions in the experimental procedure exactly. Remove obstacles by keeping lockers closed, lab stools out of aisles, and backpacks and coats stored on coat rack. For unassigned lab work, you must have the approval of the instructor. Carefully follow hazardous waste disposal instructions given later.
9. Safety Violations: Any student who does not follow the above guidelines will be given one warning and will then be removed from the lab for the day for any subsequent violations. There may also be grade deductions or permanent removal from the lab for serious violations.
What to Do In Case Of Accident
1. During your first lab period, locate the position of the fire extinguisher(s), eyewashes, safety shower, first aid kit, spill kit, phone, fire alarm pull stations, exits, hallway showers, and any other safety equipment.
2. In all cases of accident or injury, notify the TA and the instructor.
3. For any serious fire or injury: Call the POLICE DEPARTMENT (33000) from any campus phone. Security is in the best position to summon fire or ambulance service. Call the Flagstaff Fire Department (8-774-1414) or dial 8-911 if Security cannot be reached. Use the FIRE ALARM PULL STATIONS (red box by every stairwell entrance) to clear the building of personnel. THE LOCAL FIRE ALARM IN THE LAB SCIENCE FACILITY WILL SUMMON HELP BUT STILL ALWAYS CONTACT CAMPUS SECURITY FROM A SAFE LOCATION. THIS IS NOT CASE IN BLD 20 SO ALWAYS CALL SECURITY.
4. In case of a small fire: Immediately get help from your TA or instructor. Fire extinguishers are rated for ABC type fires in chemistry where A is combustible (paper, etc.), B is flammable liquids, C is electrical, and D is combustible metals. Use dry sand for D type fires or a special extinguisher rated for these fires. To use an extinguisher remember “PASS”: Pull the pin, Aim the hose, Squeeze the handle, and Sweep the base of the flames. If a person's clothing is on fire, they should immediately stop-drop-roll, use the safety shower if it is close, or smother the fire with a lab coat or fire blanket. Cover beaker fires with a watch glass or larger beaker to remove oxygen and put out the fire. Cool minor burns in cold water immediately.
5. In case of chemical contact: If the area of contact is small, flush it well under the nearest water tap for 15 minutes. Eyes must be flushed immediately using the eyewash at one of the sinks or the eyewash by the safety shower keeping the contaminated eye(s) open. In case of large areas of contact, start rinsing the person using the safety shower and remove contaminated clothing. After decontamination, the person will be taken to another shower facility if available in the building. Immediately inform the instructor or TA in any case. Buildings 17 and 88 both have shower facilities.
6. In case of mercury spillage: To dispose of this hazardous material properly, notify your TA and he or she will collect the mercury using a special spill kit.
7. Chemical spill: In case of a chemical spill notify your TA and ask for help. If the substance spilled is flammable, turn off all burners, hot plates, or electrical devices. Notify the instructor, the laboratory manager, or the stockroom manager. Clean-up materials are available in the lab or stockroom.
Hazardous Waste Disposal
The Resource Conservation and Recovery Act (RCRA) mandates the proper disposal of hazardous waste. Disposal of many waste chemicals by putting them down the sink is now illegal. Regardless of regulations, the proper management of hazardous waste is of particular importance to the people of Arizona where the contamination of groundwater by hazardous waste could have grave consequences. Please carefully follow the instructions below to protect our groundwater and keep your lab safe. Hazardous waste is determined by four properties:
TOXIC: A poisonous substance, potentially harmful to human health, can cause cancer or birth defects, or can contaminate, harm or kill wildlife.
FLAMMABLE: substances, which can explode, ignite, or emit toxic gases or fumes if exposed to a source of ignition.
REACTIVE: An unstable substance which can react spontaneously if exposed to heat, shock, air, or water. Reactions may include fires or explosions. The research director or instructor for the lab must neutralize any reactive substance before it can be accepted for disposal.
CORROSIVE: A substance that could corrode storage containers or damage human tissue upon contact. (For example, acids and bases, pH <4 or >10)
Chemical waste that does not fit into the above categories may be flushed down the drain with large amounts of water. The instructor or TA must be consulted if there is uncertainty with regard to the collection of a chemical waste.
All waste bottles are labeled and color-coded with tape. The label will include an experiment number and a hazardous waste description that will help you decide which bottles to put your waste into. Find the correct waste bottle for your experiment number and for the type of chemical waste you have; make sure the description of the composition fits the waste you are adding to the bottle. Using the wrong waste bottle could create a safety hazard and will be treated as a safety violation. The following table should help. Some nonhazardous chemical waste from experiments you do may be put down the drain. Avoid using cup sinks or water troughs to dispose of chemicals, instead use large sinks in the lab. Acidic or basic used chemicals (pH <4 or >10) will be disposed of by neutralization in a fume hood. Waste bottles may also be color-coded using the following scheme:
Blue - health hazard, poison
Red - flammable hazard, organic liquid
Yellow - reactivity hazard (strong oxidizers, etc.)
White - contact hazard, corrosive
Orange- low hazard materials with hazard codes of 2 or less
Handling Reagents
The liquids, solids, and solutions used in a laboratory are called reagents. You must become well acquainted with these reagents, their containers, and their proper use. The reagents are kept on a separate bench away from your work area. Some reagents must be kept in the fume hood because they generate flammable or toxic fumes. The reagents are grouped according to experiment, starting with Experiment 1 and ending with Experiment 7. When you need a reagent please follow these rules:
1. Be sure to use the correct reagent, especially noting the concentration. Find the reagent, check the concentration, and then carefully read the label again to be sure you have the right one. Note the hazard code and take necessary precautions.
2. Use the reagent at the reagent bench. Do not take reagent containers to your work area.
3. Please conserve and take only what you need.
4. Do not contaminate the reagents. Always use a clean spatula for solids and clean glassware for liquids. Never put a pipet, etc. into a liquid reagent, instead pour what is needed into a clean, dry container and take it to your work area.
5. NEVER return unused reagents, liquid or solid, to the reagent bottles. Discard or share any excess.
6. Put the lid back on the reagent container snugly and put it back in the correct location.
7. Clean up any reagent you spill with a wet sponge, rinse out the sponge at the sink, and then wash your hands.
8. Use great care with corrosive chemicals (strongly acidic or basic solutions). Always wear safety goggles! Rinse your hands with tap water after using corrosive chemicals, especially if you feel a burning or slimy sensation on your skin. Wear the gloves provided in the laboratory if called for. All strong acids and bases will be disposed of in the hood as noted later in experimental procedures.
9. When washing glassware, often all that is needed is to rinse well with hot tap water 4 or 5 times. If the glassware is really dirty use detergent and hot tap water. You should always rinse your test tubes, pipets, buret, volumetric flask, and other glassware out with hot tap water several times and then a small amount of pure water (RO) before storage. (Note: RO stands for reverse osmosis, one method use to purify the water. Water may also be distilled or deionized to purify it.) You do not need to dry the inside of glassware.
10. Avoid using water troughs or cup sinks to dispose of nonhazardous chemicals, instead use large sinks available in the lab. Be sure to follow the instructions in the experiments with regard to the disposal of chemicals.