NAU CHEMISTRY DEPARTMENT CHEMICAL HYGIENE PLAN
Authored by John K. Nauman
TABLE OF CONTENTS Page
INTRODUCTION 1
OUTLINE OF THE CHEMISTRY DEPARTMENT SAFETY PROGRAM 1
I. Removal of Hazards 1
II. Safety Education 2
III. Chemical Inventory Management 3
IV. Hazardous Waste Management 3
V. Emergency Preparedness 4
VI. Laboratory Safety Officer, Committee, & Director 4
THE CHEMISTRY DEPARTMENT SAFETY MANUAL 5
Appendix 1 – Forms for Use by Individual Labs 32
Lab Safety Check, Inventory Form, Record of
Safety Incident, Incident Injury Report, Emergency Phone Numbers
Appendix 2 – Glove Chart 42
Appendix 3 – Flammability Data for Common Solvents 43
Appendix 4 – Risk Assessment Chart 44
Appendix 5 – Baker Labeling and Hazard Rating Info 45
Glossary: http://www.ilpi.com/msds/ref/index.html
INTRODUCTION
Safety is a key concern in the Chemistry Department. Working with chemicals always involves an element of risk, which may be more common in the chemistry lab than in other areas, but certainly is not exclusive to chemistry. Our Chemical Hygiene Plan (CHP) is designed to prevent any unacceptable level of risk from occurring.
The safety program continues to be improved with annual updates. Robert Zoellner, Ken Bean, Wayne Hildebrandt, Don Gilbert, Mike Eastman, and Ralph Campbell deserve thanks for their work in reviewing and editing several sections of this CHP.
The CHP in the Chemistry Department has many facets. Removal of hazards, safety education, chemical inventory management, hazardous waste management, emergency preparedness, and enforcement are important elements in our safety program. These and other issues are addressed as follows by the Chemistry Department.
OUTLINE OF THE CHEMISTRY DEPARTMENT CHP
I. Removal of Hazards
The removal of safety hazards has been found to be the most effective method by which accidents can be prevented. This can be accomplished in several ways. Currently, an annual safety audit of every laboratory facility is used to identify and suggest corrective measures for unsafe situations. A safety audit is filled out for every laboratory (see Appendix 1). Hazards needing attention are noted and follow‑up action recommended. The NAU Office of Environmental Health and Safety (EH&S) implements and monitors the regular safety audit and the Chemistry Department conducts an informal audit of most labs annually. The day to day responsibility for keeping a lab safe and for follow up on a safety audit rests with the faculty or staff member in charge of a particular laboratory area (laboratory directors).
The laboratory safety officer and the laboratory manager maintain accurate records of all safety incidents or injuries in the building. A safety incident form (see Appendix 2) must be completed by the supervising faculty or staff person for injuries, including minor ones, for any students, staff, faculty, or other persons in the building. Incident report forms are available in Room 17-212. All unsafe situations must also be reported. These forms are given to the laboratory manager, who is in charge of maintaining safety incident and accident records. For any serious injuries to faculty, staff, or student employees, a Workman's Compensation "Supervisors Report of Injury" is to be completed by the department chairman or the laboratory safety officer. The employee should then visit the Student Health Center to report the injury.
Accurate records are kept for two reasons. First, safety records can be used as a guide to correct any problems that may have led to the accident. For example, these reports are used to revise experimental procedures that involve an unacceptable level of risk. Safety is a key criterion when choosing new experiments for academic laboratories. Second, these records may provide information needed in case of litigation.
Proper maintenance of equipment and facilities is also of key importance. Emergency problems such as water spills from leaking pipes should be reported by calling facilities management at 3-4227. Less urgent problems such as burned out light bulbs and leaky water taps can also be reported using this number.
Fume hoods are essential safety devices requiring special attention. Faculty and TAs are responsible for checking the fume hoods in research and teaching laboratories for proper operation before use by students.
II. Safety Education
Safety education begins with the training of student workers and researchers working in lab areas. Anyone who could be exposed to, works with, or handles chemicals must attend must attend "Chemical Hygiene OSHA Lab Training" offered by the Office of Regulatory Compliance (ORC). The EH&S also offers three other courses that are required for anyone working with Biohazards, Hazardous Materials Shipping, and Blood-borne Pathogens. Every faculty, staff, or student employee (including students doing research for credit) working in a lab area is required to read and be familiar with the Chemistry Department chemical hygiene plan. A text published by the American Chemical Society, "Safety in Academic Chemistry Laboratories" (posted on instructional labs homepage) and the NAU Laboratory Safety Standard (Chemical Hygiene Plan) should also be reviewed. All students taking chemistry labs are required to read safety information provided and watch videos on basic lab safety and the use of material safety data sheets (MSDS). They must also complete a MSDS tutorial-quiz and provide the department with a copy of the completion certificate that is kept on file. All CHM152L students are shown a video on the use of safety showers and eyewashes. CHM235L students are required to view a video on fume hood use. A record of safety training is maintained every semester on the laboratory performance contract.
A laboratory safety mini course (CHM 295 or 595) is offered every fall. All graduate and undergraduate student employees working in instructional laboratory areas must take CHM 295 or 595. Every semester, a one to two hour orientation meeting reviewing basic safety concepts, recent accidents, and changes in policy will be held. Attendance is required of all students working in lab areas. Student researchers are required to attend one orientation per year, and it is suggested that they attend both meetings and take CHM295/595. More information on training requirements for student employees and research students is provided in the department safety manual.
Safety training for students begins on the first day of each instructional laboratory. A safety lecture is given during the first meeting in every laboratory section. There is a section on safety in the introduction of all in-house laboratory manuals for academic laboratories. There is additional information at the start and/or in the procedure of every experimental write-up covering specific hazards for that experiment. If the laboratory class does not use an "in house" laboratory manual, a safety handout is provided on the instructional lab’s home page. This handout includes information on safety rules, chemical first aid, emergency procedures, hazard codes, hazardous waste disposal, etc. All laboratory students are required to read and study the safety material provided. On the back of every locker list is a safety assignment that must be completed and signed by every student.
Safety and safety training is the responsibility of all faculty and staff of the Chemistry Department. However, faculty and staff members who supervise activity in academic and research laboratories have primary responsibility for safety and safety training in their laboratories. Certain safety rules must be followed and enforced by faculty and staff who work and supervise students in laboratory areas. These rules are listed later in this document.
III. Chemical Inventory Management
Chemical inventory management is an important issue. The following guidelines should be observed for the purchase of chemicals:
A. Inventories of chemicals are maintained at low levels and small quantities are purchased whenever possible.
B. Only items regularly used (once a year or more) are ordered routinely.
C. Flammable liquids are ordered in one liter containers or less except for high‑use solvents. High‑use solvents purchased in bulk are stored in an exterior solvent shed or storage cabinets for flammable liquids less than the storage limits of the building fire code.
D. Extremely toxic or reactive chemicals or those with a shelf-life of less than one year are ordered only as needed.
E. An accurate inventory listing of all chemicals stored in the stockroom is maintained. In addition an annual inventory of all the chemicals in other chemistry lab areas is also done. The chemistry office, campus police, and the EH&S are provided with updated printouts annually.
F. All chemicals, upon receipt, are checked for proper safety information on the label and labeled with the date received and the expiration date when possible. Material safety data sheets for new chemicals are filed in Room 17-212 and the lab room where the product or chemical used.
IV. Hazardous Waste Management
Since the Chemistry Department generates significant amounts of hazardous chemical waste, the department has addressed the problem in two ways: by limiting production and by developing a hazardous waste plan.
Ordering small amounts of chemicals reduces the amount of hazardous waste generated by the department. The chemicals used in academic labs are carefully chosen. When possible, experiments producing large amounts of hazardous waste are modified to reduce volume of waste generated or to use less hazardous reagents. Special attention needs to be paid to decreasing the use of mercury compounds (or avoiding use completely), since disposing of mercury waste is significantly more costly. The generation of hazardous waste is a critical issue when selecting new experiments.
Unfortunately where hazardous waste is generated, it must be collected in a very organized manner. Waste is collected so that the chemical constituents and their approximate amount (percent) in each waste bottle will be known. Chemical waste is segregated into different bottles to prevent mixing of incompatible chemicals. In order to address this complex issue, an instruction manual has been written and is included in the Chemistry Department safety manual.
Follow‑up is important with regard to hazardous waste collection. An informed student is assigned to instructional laboratories to collect hazardous waste weekly and to check for proper collection procedures. In research laboratories proper hazardous waste collection is the responsibility of a lab's research director. Full tagged waste bottles in research laboratories are disposed of by calling the EH&S and arranging for them to pickup the bottles. Full waste bottles are never to be taken to the stockroom except by the student employee assigned to do so or by the laboratory manager.
V. Emergency Preparedness
Even in the event that all of the safety measures above are followed, certain emergency preparations need to be addressed, such as response to emergencies and training in the use of emergency equipment. The issue of training is addressed at the beginning of every semester during student employee meetings. Emergency procedures and first aid are covered at these meetings. These emergency procedures are outlined in the department safety manual.
In the event of an emergency, necessary equipment must work. The Chemistry Department, Facilities Services, and/or Environmental Health and Safety check safety showers, eyewashes, AEDs, fume hoods, and other safety equipment as required. The fire extinguishers are maintained by Fire Life Safety. Any problems are corrected as soon as possible.
VI. The Laboratory Safety Officer, Safety Committee, & Directors
Before describing the roles of the laboratory safety officer, safety committee, and laboratory directors, we must realize that safety is a shared responsibility. University administrators, faculty, staff, and students must all be part of a viable safety program. Without the support of the administration and the students, a safety program cannot be effective.
There are, however, certain roles that must be filled to get the job done. The role of the laboratory safety officer position is to promote, monitor, and help enforce departmental policy. The laboratory safety officer reports to the department chairman and coordinates safety activities at the departmental level. The laboratory safety officer is chosen by the department chairman from faculty and staff members of the Chemistry Department and represents the Chemistry Department in matters of safety.
The department safety committee and chairman are chosen from chemistry faculty and staff and provide overall departmental representation. The laboratory safety officer and safety committee have the responsibility for developing departmental safety policy and helping to enforcing this policy. Any unresolvable concerns will be forwarded to the department chairman for resolution. Problems not solved by department action will be forwarded to the Dean of the College and the EH&S.
Laboratory directors (faculty) have the responsibility for specific aspects of the department safety program such as lab-specific training and standard operating procedures, hazardous waste collection, chemical management and inventories, etc. for their research and upper division instructional laboratory areas.
CHEMISTRY DEPARTMENT SAFETY MANUAL
TABLE OF CONTENTS Page
Introduction 5
I. Training Requirements 5
II. Safety Rules or How to Protect Yourself 6
III. Chemical Labels and Hazard Codes 9
IV. Safety References and Material Safety Data Sheets 13
V. Fire Safety 14
VI. Hazardous Waste Collection 15
VII. The Use and Management of Chemicals 20
VIII. Proper Use of Fume Hoods 24
IX. Cleaning Glassware 25
X. Handling Reagents 25
XI. Radiation Safety 26
XII. Laboratory Hazards and Safety Information Outline 26
XIII. What To Do in Case of an Accident or Safety Problem 28
XIV. Emergency Procedures 30
XV. Environmental Monitoring 32
XVI. Medical Monitoring. 32
Appendix 1 – Forms 32
Special Forms:
Laboratory Safety Audit 33
Chemical Inventory Form 36
Record of Safety Incidence 37
Incidence Injury Report 39
Emergency Numbers to Post 41
Stockroom Authorization Form 42
INTRODUCTION
Safety is a key concern in the Chemistry Department. Working with chemicals always involves a certain element of risk, which may be more common in the chemistry lab than in other areas, but certainly is not exclusive to chemistry. As instructors, researchers, and employees in the Chemistry Department we must be aware of safety at all times and set a good example for our peers and those people we supervise. A central theme of this manual is to always be informed and alert when working in the laboratory environment. Laboratory safety is much like driving a car in that when we take it for granted and stop paying attention, sooner or later we pay the price for our laxity.
The first part of this manual will focus on safety protocols to lower the probability of a safety incident. The latter part of this manual will deal with aspects of response to common safety problems, which could occur even when preventive measures are followed. This manual is to be used in conjunction with the ACS publication, "Safety in Academic Chemistry Laboratories". This manual will only cover department policy regarding safety; not every aspect of safety is covered. Information on safety is available in the MSDS - TA Training Room (Room 212) to answer additional questions on safety issues.
I. TRAINING REQUIREMENTS
Safety education begins with the training of faculty, staff, and students. In addition to the training described on page 2, each semester a one hour orientation meeting with safety as its primary focus is held. Attendance is required of all students working in laboratory areas. Student researchers are strongly encouraged to attend. Safety training records for lab courses and student employees (teaching assistants and stockroom workers) will be maintained by the laboratory manager.
II. SAFETY RULES (HOW TO PROTECT YOURSELF)
The following rules need to be followed by any person working in the laboratory environment of the Chemistry Building:
A. Students are not to do lab work alone after 5:00 pm or on weekends. Someone else must always be in the lab or be very close by in case of an emergency. Notify coworkers when conducting hazardous experiments.
B. Complete notebook records are to be kept for all work. All laboratory activities are to be related to the research projects or work assignments only.
C. Telephones are located in most research labs and house phones can be found in the north and south 2nd floor modules of Building 17 and in Common Area. Emergency phones are located in the north hallway of Building 20 in red boxes. Each person working in a laboratory must be aware of the use and location of the nearest fire extinguisher(s), safety shower, eye washes, AED, first aid kit, etc.
D. All chemicals checked out of the stockroom by a research student require the signed authorization of the supervising professor. Teaching assistants (TAs) and stockroom workers may check out chemicals related to their work only. Chemicals will not be checked out to students in an academic laboratory class without the written approval of the instructor (a signed and completed requisition form).
E. Eye protection (indirectly or nonvented goggles) is required at all times when in a laboratory.
Exceptions to this requirement are:
1. A research student who is working at a desk in a research laboratory if no experiment involving chemicals or other hazards is in progress.
2. In an instructional laboratory during a "lab-lecture" if no experiments are in progress and if the instructor or TA has announced that eye protection is not required at that time.
3. A student working in the chemistry stockroom must wear goggles any time chemicals are being handled in the stockroom. Approved safety glasses must be worn whenever goggles are not needed but some form of eye protection must be worn at all times in the prep stockroom areas (Room 216).
F. Whenever large volumes of corrosive liquids such as base baths, "Nochrom Cleaning Solution", acids, bases, etc. are prepared, used, moved, or transferred, secondary containment is required along with more personal protection. A lab apron, special shoes, larger gloves, goggles, and perhaps a face shield should be used. If needed, thick walled Erlenmeyer flask(s) should also be used.
G. Cleaning Solutions will only be prepared and stored in sulfuric acid bottles with a shatter proof plastic coating. Vented caps must be used with this solution. The volume should not exceed 2.5 liters. This bulk solution will only be used to fill pint bottles with vented caps. Always use less of any corrosive chemical and use small dispensing containers whenever possible.
When preparing or refreshing "Nochrom Cleaning Solution," add the solid Nochrom powder slowly when mixing. The bottle holding the Nochrom solution can be stirred by placing it in a plastic tub sitting on a wide magnetic stirrer. The plastic tub will contain the solution if it foams out of the bottle. Do not put the lid back on until you are sure it has stopped reacting.
Pipets will be cleaned in an ultrasonic bath or if this fails, they are filled with cleaning solution from a pint bottle of Nochrom solution and placing them in plastic trays. After several minutes this solution will then be drained back into the pint bottle. The pipets will then be rinsed at least five times in the sink with tap water inside and out and then once with distilled water checking to see that pipets are clean as they are drained. Pipets requiring additional cleaning will be handled in another location. Full time staff or faculty member should supervise volumetric glassware requiring more additional cleaning.
An alcoholic base bath used for cleaning must be labeled correctly and kept closed since it is hazardous (flammable, etc.). Employees using a base bath must take the same precautions as when using "Nochrom Cleaning Solutions" and also wear long neoprene gloves and perhaps a face shield. This will also be the case for an acid cleaning bath.
H. Material Safety Data Sheets (MSDS) and other safety literature are available in Room 17-212. All students must be made aware of the location and use of MSDS before they use hazardous chemicals. When possible, copies of MSDS for all chemicals in a lab should be kept in that laboratory area. The MSDS must be read before using any chemical with a hazard code containing a "3 or 4" hazard or if the hazard code is unknown. The MSDS is suggested reading for any chemical with a hazard rating of "2" or if you are unfamiliar with that chemical.
I. Any chemical or reagent prepared, used, and/or stored in a lab must be labeled with the name and amount of each compound present, the date it was prepared, and the name of the person who prepared it. Any special hazards or precautions must also be noted on the label. Secondary containment must also be used.
J. Electrical cords should be replaced if damaged and kept off the floor where there are safety showers nearby or areas likely to flood.
K. As a portion of the safety training in instructional laboratories, the following rules and guidelines will be distributed (in the syllabus, manual, or separately) and discussed. Note: Teaching assistants need to avoid becoming authority figures when enforcing these safety rules. Instead, simply relay the rules to the students. For example, you might say, "federal and state regulations require that eye protection be worn at all times in laboratory areas." If enforcement becomes a problem, let the instructor be the authority figure. This will help preserve your role in the lab as a helpful resource and peer-mentor.
L. Standard Lab Polices and Rules Enforced in all Instructional Labs:
Laboratory safety involves the prevention of and response to laboratory emergencies. Good prevention is far better than someone getting hurt. This begins with always being aware of chemical and laboratory hazards. Hazard codes, chemical labels, and material safety data sheets are the first sources of information that help us prepare to work safely in a laboratory. This information can be used to do risk assessment on the hazards and precautions for the experiment you are about to do. Certain rules need to be followed to keep you safe and you must know what to do in case of an emergency. Chemical waste management is another important aspect of a safe laboratory and a key regulatory compliance issue.
Risk Assessment
A risk assessment determines what hazards will be encountered during an experiment or lab procedure, how to mitigate them (precautions such as goggles or gloves), and what should be done if something goes wrong. There may be physical or chemical hazards present that will be discussed in the experiment write up. Chemical hazards will be expressed using hazard codes and/or special warning stickers on bottle labels. If you observe a 3 or 4 in the hazard code you may want to obtain more information by referring to the material safety data sheet (MSDS) and note hazards and how to respond to them. For every experiment you must write a risk assessment and outline the experimental procedure before you start lab work.
1. EYE PROTECTION MUST BE WORN IN THE LABORATORY AT ALL TIMES unless otherwise notified by the instructor or TA. Avoid rubbing your eyes in lab unless you wash your hands first. Use extra caution when using corrosive chemicals. Indirectly vented or nonvented goggles are the required eye protection for this lab course. Safety glasses or directly vented goggles are not acceptable. Do not modify or remove the vents on goggles. Write your name and section letter on your goggles.
2. Skin protection should be employed where appropriate; you may be required to wear long pants. Avoid wearing shorts. The use of a lab coat or plastic apron is recommended. Closed toed shoes must be worn at all times in the laboratory for protection against broken glass and spilled chemicals. Open-toed shoes or sandals are not appropriate footwear in lab areas. Disposable gloves are available for the handling of hazardous chemicals. Always remove them before exiting the lab. After completing lab work for the day, wipe down your entire work area (or any area used include balance, fume hoods, or reagent areas) with a clean damp sponge to clean up any spilled chemicals and other material. Rinse out the sponge several times and wring it out. Wash your hands as you exit the lab.
3. Protection from fumes or fine powders: Never allow hazardous chemical fumes or dust to escape into the open room; use fume hoods when necessary or specified. Be sure to use the fume hoods correctly, following the instructions provided by your TA or instructor. Avoid putting your head inside the fume hood and close the sash or fume hood window when it is not in use.
4. Protection from internal poisoning: Never "pipet by mouth", eat, drink, or smoke in the laboratory. These activities are prohibited. Wash your hands after you have completed lab work or leave the lab room.
5. Protection from hot surfaces: Use the appropriate types of tongs to handle hot objects. Test tube holders are too weak for carrying flasks.
6. Protection from fire and explosion: Never allow flammable vapors to escape into the open room (see No. 3). Ether is especially dangerous in this respect. Never use an open flame while flammable liquids are being used in the room. Hot plates should be used with care, as they are an ignition source. Flammable volatile liquids should be used in fume hoods and stored in solvent cabinets when possible. Long hair should be tied back to keep it away from open flames.
7. Protection from cuts: When manipulating glassware or ceramic ware, protect your hands with a cloth towel. Clean up broken glass immediately. Do not pick up broken glass with bare hands. Use a broom and dustpan to dispose of glass in the "Broken Glass Container." Do not clean up broken mercury thermometers without help from your TA since mercury requires special disposal procedures.
8. Protection from the unexpected: Always read all labels noting the chemical name, formula, concentration, and warnings (including hazard codes) carefully and double check to make sure you have the correct chemical and concentration. Follow directions in the experimental procedure exactly. Remove obstacles by keeping lockers closed, lab stools out of aisles, and backpacks and coats stored on coat rack. For unassigned lab work, you must have the approval of the instructor. Carefully follow hazardous waste disposal instructions given later.
9. Safety Violations: Any student who does not follow the above guidelines will be given one warning and will then be removed from the lab for the day for any subsequent violations. There may also be grade deductions or permanent removal from the lab for serious violations.
III. CHEMICAL LABELS AND HAZARD CODES
The hazards of a chemical must be known before it is used. The label on the chemical container is the first source of information. At a minimum, the label must always include the name and concentration of every chemical in the bottle (don't forget the solvent). Other information such as the date prepared, hazard code or warnings, and preparer's initials are required. Chemical manufacturers also provide information such as chemical hazards, recommended protective equipment, storage codes, and physical data on the label.
The Chemistry Department has adopted the “Baker” hazard code classification system to inform users of potentially hazardous chemicals. This system is designed to provide information to people who handle chemicals in laboratories. Hazards are classified according to four types: health (toxic), flammability (fire), reactivity (explosive or reactive), and contact (corrosive). The intensity of the hazard is indicated by using a number from "0" (no hazard) to "4" (extreme hazard). This information is conveyed using either a four-colored label found on "J.T.Baker" chemical products or a series of four digits. The label on chemical bottles may look like this:
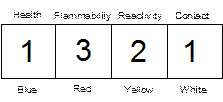
The four-digit hazard code may also be listed as follows in lab manuals, chemical inventories, etc.: 1321
For example, the codes listed above for acetone indicate a slight health hazard (1), a high flammability hazard (3), a moderate reactivity hazard (2), and a slight contact hazard (1). Hazard codes will be listed after the chemical inside parenthesis: (1321)
The "Baker Codes" for each of the four hazards are defined according to the following scheme:
HEALTH (BLUE): Toxic effects of a substance if inhaled, ingested, or absorbed.
0. No Hazard
1. Slight hazard
2. Moderate hazard
3. Severe danger
4. Deadly, Life Threatening
FLAMMABILITY (RED): Tendency of a substance to burn.
0. Will not burn
1. Flash point above 200°F
2. Flash point between 100-200°F
3. Flash point between 73-100°F
4. Flash point below 73°F
REACTIVITY (YELLOW): Potential of a substance to react violently with air, water or other substances.
0. Stable
1. Reacts under elevated temperature or when in contact with other substances under abnormal working conditions
2. Reacts violently but will probably not explode under normal working conditions
3. Reacts violently or explodes under normal working conditions when in contact with air, water or other substances
4. May react violently or detonate spontaneously under normal working conditions
CONTACT (WHITE): The danger a substance presents when it comes in contact with skin, eyes or mucous membranes.
0. No contact hazard to normal, healthy tissues
1. Slight hazard; irritant to sensitive tissues, avoid contact with eyes and mucous membranes
2. Moderate hazard; irritant to sensitive tissues, damages tissues.
3. Severe danger; destroys tissues, including skin
4. Extreme danger; life threatening
The National Fire Protection Association (NFPA) has a hazard code system that was adopted in 1975 to communicate hazards in a fire, spill, or general emergency situation. This system uses a diamond-shaped label with which you may be familiar, since it appears on entrances to stores containing hazardous chemicals and on chemical containers. The NFPA may differ from the “Baker” code since it provides information to firefighters, while the “Baker” code provides hazard information in a laboratory situation. The codes are very similar except the white section in the NFPA code refers to special or specific hazards of importance to firefighters such as “ox” for oxidizing agent.
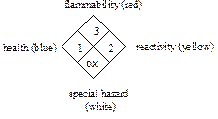
The positions on the NFPA diamond are defined as follows:
Health Hazard (Blue): Degree of hazard for short-term protection
0. Ordinary combustible hazards in a fire
1. Slightly hazardous
2. Hazardous
3. Extreme danger
4. Deadly
Flammability (Red): Susceptibility to burning
0. Will not burn
1. Will ignite if preheated
2. Will ignite if moderately heated
3. Will ignite at most ambient conditions
4. Burns readily at ambient conditions
Reactivity, Instability (Yellow): Energy released if burned,
decomposed, or mixed
0. Stable and not reactive with water
1. Unstable if heated
2. Violent chemical change
3. Shock and heat may detonate
4. May detonate
Special Hazard (White position on diamond):
OX. Oxidizer
W. Use no water, reacts!
The American Coatings Association developed the Hazardous Materials Identification System, HMIS. This system was created in order for better compliance with OSHA’s HCS 29 CFR 1910.1200.
The HMIS components are defined as follows:
Health Hazard (Blue): Degree of hazard for short-term protection. If an asterisk is present this represents a chronic health hazard if long term exposure occurs.
0. No signification risk to health.
1. Irritation or minor reversible injury possible.
2. Temporary or minor injury may occur.
3. Major injury likely unless prompt action is taken and medical treatment is given.
4. Life-threatening, major or permanent damage may result from single or repeated overexposures.
Flammability (Red): Identical to the NFPA system.
0. Materials that will not burn.
1. Materials that must be preheated before ignition will occur. Includes liquids, solids, and semi solids having a flash point above 200 degrees F.
2. Materials, which must be moderately heated or exposed to high ambient temperatures before ignition will occur. Includes liquids having a flash point at or above 100 degrees F but below 200 degrees F.
3. Materials capable of ignition under almost all normal temperature conditions. Includes flammable liquids with flash points below 73 degrees F and boiling above 100 degrees F, as well as liquids with flash points between 73 degrees F and 100 degrees F.
4. Flammable gases, or very volatile flammable liquids with flash points below 73 degrees F, and boiling points below 100 degrees F. Materials may ignite spontaneously with air.
Physical Hazard (Orange): There are seven classes of physical hazards: water reactives, organic peroxides, explosives, compressed gases, pyrophoric materials, oxidizers, and unstable reactives.
0. Materials that are normally stable, even under fire conditions, and will not react with water, polymerize, decompose, condense, or self-react. Non-explosives.
1. Materials that are normally stable but can become unstable (self-react) at high temperatures and pressures. Materials may react non-violently with water or undergo hazardous polymerization in the absence of inhibitors.
2. Material that are unstable and may undergo violent chemical changes at normal temperature and pressure with low risk of explosion. Materials may react violently with water or form peroxides upon exposure to air.
3. Materials that may for explosive mixtures with water and are capable of detonation or explosive reaction in the presence of a strong initiating source. Materials may polymerize, decompose, self-react, or undergo other chemical change at normal temperature and pressure with moderate risk of explosion.
4. Materials that are readily capable of explosive water reaction, detonation or explosive decomposition, polymerization, or self-reaction at normal temperature and pressure.
HMIS labeling schematics:
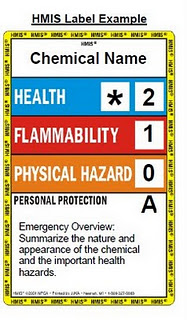

When any digit of a hazard code is a three or four, the Material Safety Data Sheet (MSDS) must be read to obtain additional safety information before the chemical is used. These sheets are available in Room 17-212 for all chemicals used in the Chemistry Department; they must not be removed from that room.
The hazard codes are given only as a guide to warn the user of probable hazards and to approximate the degree of hazard under normal use. The user must not be lulled into a false sense of security by a low number on the label, but must take full responsibility for safe use of the chemicals. Avoid over-reliance on hazard codes. Refer to the Material Safety Data Sheets (MSDS) and other safety information whenever you are working with chemicals that are unfamiliar to you. This is especially important when mixing chemicals. Chemicals with relatively safe hazard codes can become dangerous when mixed with other chemicals.
IV. SAFETY REFERENCES AND MATERIAL SAFETY DATA SHEETS
Often the label on a chemical container does not provide enough information on a chemical to use it safely. Additional facts on chemical hazards can be found in material safety data sheets, safety references, and chemical/safety catalogs. A safety library is maintained in Room 17-212. Material Safety Data Sheets (MSDS) for every chemical purchased by the Chemistry Department are maintained and use of these sheets is encouraged. Aldrich/Sigma MSDS volumes are also available as are extra copies of some MSDSs. You can also search the chemical name and MSDS to quickly find information.
The Federal Government is in the process of adopting the new Global Harmonized System (GHS) using a more uniform hazard communication system that will be implemented nationally in the near future. In the GHS the Safety Data Sheet (SDS) will replace the MSDS.
Other references on laboratory safety, hazardous chemicals, carcinogens, chemical first aid, exposure limits, hazardous waste disposal, etc. are available in Room 17-212. The following references should be familiar to all researchers and other employees: The NAU Chemical Hygiene Plan and the NIOSH/OSHA Pocket Guide To Chemical Hazards
The following references are also very good:
· CRC Handbook of Laboratory Safety
· Chapters 4 and 5 of "Safety In Working With Chemicals" by Green and Turk
· "Dangerous Properties of Industrial Materials" by Sax
· Toxic Substances List (NIOSH)
· Annual Reports on Carcinogens
· Emergency Response Guide
· "First Aid Manual for Chemical Accidents" by Lefevre
Free sources of information such as chemical catalogs (the Baker, Aldrich and Flinn catalogs are good references) and safety catalogs can provide information such as the right choice of gloves for a certain class of hazardous compounds (available in Room 212). Room 212 in Building 17 is open Monday through Friday from 8:00 am to 5:00 pm and Tuesday evening to make this safety material more available. This material can be removed from Room 212 for making copies for only 30 minutes or less.
The Material Safety Data Sheet (MSDS) provides technical, chemical, physical, and hazard information for the "hazardous material." The "Hazardous Material" may be an individual substance or a mixture of hazardous ingredients. The Occupational Safety and Health Administration (OSHA) require manufacturers to prepare an MSDS for each hazardous substance they make. While OSHA is very specific about the information that must be provided in an MSDS, they do not require manufacturers to provide the information in a certain format. Therefore, the order in which information is provided in a MSDS may vary from manufacturer to manufacturer. The following is an outline of the content of a MSDS, but not necessarily in the order provided by all manufacturers:
· Material Identification and Hazardous Components
· Physical/Chemical Characteristics - vapor pressure, flash point, etc.
· Fire and Explosion Hazard Data - auto ignition temperature, extinguishing media, etc.
· Reactivity Hazard Data - water and air reactivity, incompatibility, etc.
· Health Hazard Data - TVL, PEL, etc.
· Control and Protective Measures - the type of personal protective equipment and type of ventilation to be used, and the precautions to be taken when using the material for its intended purpose is given
· Precautions for Safe Handling and Storing - use/leak procedures
Please note that more sections and more information may be provided by the individual manufacturer; however, the information required in the listed sections MUST be found in a MSDS. While all of the information in the MSDS is important, the information on safe handling, control and protective measures, reactivity/health hazards, and extinguishing media is very important. When using a MSDS, keep in mind that the target audience is assumed to be a chemical worker using larger amounts of the material than you will encounter. The personal protection measures may sometimes need to be modified for a laboratory situation where much smaller amounts are used in a more controlled environment. For the safety of yourself and those people with whom you work be sure to read the MSDS on any chemical you work use. BE INFORMED! Read labels and use care when using concentrated reagents. A web site for excellent MSDS info and general safety information is: http://www.ilpi.com/msds/index.html this site also has a very useful MSDS quiz and a glossary of terms.
V. FIRE SAFETY
A. GENERAL INFORMATION
In most chemistry laboratories all hallways are equipped with a fire extinguisher. There have not been any damaging or large fires in several years in the Chemistry Department probably due to a stressing on common sense, preparation, and a little luck. There have been some small fires on lab benches. These small fires did not get out of control or cause damage for several reasons:
1. Preparing in advance by knowing:
2. The hazards of chemicals in use
3. The location of extinguishers and safety showers and their use
4. Always using less and storing bulk solutions in storage cabinets for flammables
5. Using common sense in putting out fires
6. If the fire is contained in a beaker or flask put it out using a watch glass, larger beaker, etc. to cover it and remove the oxygen supply
7. If the fire is not confined inside a container then use a fire extinguisher only if it is safe to use
8. To use an extinguisher:
9. Select proper extinguisher for fire (explained later)
10. Twist pin and pull
11. Hold the extinguisher in upright position and aim the hose or bell at base of the flames from a distance
12. Squeeze handle until extinguishing material is released
13. Slowly approach fire sweeping the base of the flames
14. Continue until fire is out
15. Continue to watch for auto ignition even after fire appears out
16. In summary, remember "PASS"; Pull, Aim, Squeeze, and Sweep
B. BASIC ELEMENTS OF FIRE
1. INTRODUCTION- Fire burns because three components are present- heat, fuel and oxygen. Fire is a chemical reaction. It happens when a material unites with oxygen so rapidly that it produces flame. Think of fire as a triangle. If any one of the three sides, heat, fuel or oxygen, is taken away, the fire goes out. This is the basis for fire extinguishment. Heat can be taken away by cooling, oxygen can be taken away by excluding air, fuel can be removed to a place where there is no flame, chemical reaction can be stopped by inhibiting the oxidation of the fuel.
2. REMOVE HEAT- Cooling a fire calls for the application of something that absorbs heat. Although there are others, water is the most common cooling agent. Water is commonly applied in the form of a solid stream, finely divided spray or incorporated in foam.
3. REMOVE FUEL- Often, taking the fuel away from a fire is difficult and dangerous, but there are exceptions. Flammable liquid storage tanks can be arranged so their contents can be pumped to an isolated empty tank in case of fire. When flammable gases catch fire as they are flowing from a pipe, the fire will go out if the flow can be valved off.
4. REMOVE OXYGEN- Oxygen can be taken away from a fire by covering it with a wet blanket, throwing dirt on it or covering it with chemical or mechanical foam. Other gases heavier than air, such as carbon dioxide or a vaporizing liquid, can be used to blanket the fire, preventing the oxygen from getting to the fire.
5. STOP THE REACTION- Studies made during recent years have indicated that the familiar statement, "Remove heat, remove fuel or remove oxygen, to extinguish a fire" does not apply when dry chemical or halogenated hydrocarbons are used as the extinguishing agents. These agents inactivate intermediate products of the flame reaction resulting in a reduction of the combustion rate [the rate of heat evolution] and extinguishing of the fire.
C. CLASSIFICATION OF FIRES
1. CLASS ["A"] fires occur in ordinary combustible materials such as wood, cloth and paper. The most commonly used extinguishing agent is water, which cools and quenches. Fires in combustible materials are also extinguished by special dry chemicals for use on Class A, B and C fires. These provide a rapid knock down of flame and form a fire retardant coating, which prevents flash.
2. CLASS ["B"] fires occur in the vapor-air mixture over the surface of flammable liquids such as grease, gasoline and lubricating oils. A smothering or combustion inhibition effect is necessary to extinguish Class "B" fires. Dry chemical, foam, vaporizing liquids, and carbon dioxide all can be used as extinguishing agents depending on the circumstances of the fire.
3. CLASS ["C"] fires occur in electrical equipment where non-conducting extinguishing agents must be used. Dry chemical, carbon dioxide, and vaporizing liquids are suitable. Because foam, water (except as a spray), and water-type extinguishing agents conduct electricity, their use can kill or injure the person operating the extinguisher, and severe damage to electrical equipment can result.
4. CLASS ["D"] fires occur in combustible metals such as sodium, potassium, lithium, magnesium, titanium, or zirconium. Specialized techniques, extinguishing agents and extinguishing equipment have been developed to control and extinguish fires of this type. Normal extinguishing agents generally should not be used on metal fires, because there is a danger in most cases of increasing the intensity of the fire because of a chemical reaction between some extinguishing agents and the burning metal. Since the Chemistry Department doesn't have a Class "D" fire extinguisher, dry sand or graphite can be used to smother Class "D" fires. Buckets of dry sand should be provided in labs where Class "D" fire hazards are present.
D. TYPES OF FIRE EXTINGUISHERS IN THE CHEMISTRY DEPARTMENT AT NAU
1. Dry chemical ABC extinguishers, which have a pressure gauge and smaller hose opening, are the most effective and can be used on all types of fires except Class D.
2. Carbon dioxide (CO2) BC extinguishers do not have gauges and have wide nozzles. They should be used on B or C class fires and are less effective on Class A fires. They should not be used on Class D fires.
3. Clean agent halo ABC extinguishers found inside labs in Building 17 are an alternative to dry chemical, halon, or CO2 type extinguishers.
4. When possible a CO2 or halon extinguisher should be used on fires in computer or instrument rooms. Dry chemical extinguishers will leave a residue capable of affecting electronic components.
5. There are two Class D fire extinguishers in Building 17, one in 212 and one in Dr. Hurst's research lab (226). These may only be used on class D fires and special training is required to use them. Graphite or dry sand can also be used to put out class D type fires.
E. CHARACTERISTICS OF FLAMMABLE LIQUIDS
Flammable liquids always generate vapor due to their high vapor pressure. When mixed with air and contacted by an ignition source, it is the vapor, not the liquid, which burns. The fuel vapor and oxygen provide two sides of the fire triangle. A flammable liquid is usually more dangerous when temperatures are high because more vapors are generated. The following terms are used to describe the safety characteristics flammable liquids:
1. FLASH POINT: The lowest temperature at which a liquid still has adequate vapor pressure to give off enough vapor to form a flammable mixture with air that will ignite with a spark.
2. FIRE POINT: The lowest temperature at which the vapor-air mixture will continue to burn after it is ignited. This is generally a few degrees above the flash point.
3. IGNITION TEMPERATURE: The temperature at which a mixture of flammable vapor and air will ignite without a spark or flame. This term is also applied to the temperature of a hot surface, which will ignite flammable vapors. The temperature varies with the type of surface.
4. FLAMMABLE OR EXPLOSIVE RANGE: The range between the smallest and largest amounts of vapor in a given quantity of air, which will explode or burn when ignited. The amount is usually expressed in percentages. For instance, carbon disulfide has an explosive range of one to 50 percent. If air contains more than one or less than 50 percent of carbon disulfide vapor, the mixture can explode or burn.
5. FLAMMABLE: A flammable is a substance with a flash point less than 100°F.
6. COMBUSTIBLE: A combustible is a substance with a flash point greater than 100°F.
7. STATIC SPARK HAZARD: When transferring flammable substances from one container to another it is important to ground both containers to prevent static sparks.
8. GASOLINE AS A FIRE HAZARD: The most commonly used flammable liquid is gasoline. It has a flash point of about -50°F (-46°C). The ignition temperature is about 495°F (257°C), a comparatively low figure. Burning gasoline has a temperature above 1500°F (800°C). Therefore, it can heat objects in the fire area above their ignition temperature. To prevent reignition after extinguishment, the agent should be applied for sufficient time to allow hot objects in the fire area to cool below the ignition temperature of the gasoline. The flammable range of gasoline is only 1.3% to 6%. Gasoline vapors are heavier than air. They tend to flow downhill and downwind from liquid gasoline, making it possible for explosive mixtures to collect in low points such as pipe trenches or terrain depressions. If the amount of oxygen in a given atmosphere is reduced from its normal 21 percent to 14 percent by diluting with carbon dioxide, most petroleum products cannot burn. As a result, a gasoline fire can be "suffocated" by diluting the atmosphere with an inert gas. It is dangerous to use water in a solid stream on a gasoline fire because it may spatter the fuel or raise its level in a container so it overflows.
A LAST WORD-- Classification of fires is important. It determines the way a fire must be put out. Smoldering embers must not be allowed to remain after a Class "A" Fire. Use of water could spread Class "B" fires such as gasoline or other flammable liquids. Conductors of electricity, such as water, should not be used on a Class "C" fire; and Class "D" combustible metals require special types of extinguishing agents since water, CO2, and dry chemical will only feed a "D" fire.
VI. HAZARDOUS WASTE DISPOSAL
Hazardous waste collection and disposal is a crucial aspect of laboratory safety. If used chemicals are determined to be hazardous, the key to safe chemical waste collection is careful planning, labeling, and collection. The contents of every waste bottle must be known to prevent mixing of non-compatible waste and allow for safe disposal. Three methods are used to collect hazardous waste or dispose of used chemicals.
1. Labels Only: Any procedure or experiment repeated several times which generates waste with a consistent composition should have a waste bottle set aside for it. This method should be used whenever possible in both research and instructional labs. Only hazardous waste labels and colored tape described later are required for labeling waste of known composition. An example label might look like:
White and Blue Tape Above Label
|

NOTE: Since the percent of each component is listed on the label, we do not need to use a log sheet when waste is collected in this way. This way of collecting waste is the collection method of choice whenever possible.
2. Labels and Log Sheets: When different procedures generate an inconsistent variety of chemical waste a hazardous waste log must be kept for each generic waste bottle (label does not give an exact waste description but might say "Waste Simple Organics"). This method is sometimes useful in research labs.
3. Bulk Collection of Waste: This would be related to Method 1, but when large volumes of nearly the same composition of used chemicals or mainly one chemical is collected over an extended period, it can be added to a bulk container on site for pickup by Regulatory Compliance. This method is currently used by CHM 151L, 235L, 238L and some research labs.
a. To setup this type of collection process, contact the Regulatory Compliance and provide them with the composition of the waste stream and assigned name for each bulk category.
b. For example, the waste stream for CHM235L is now composed of "Acetone Waste", "Aqueous Waste" and "Solid Organic Waste" where the waste for first two is collected in 2.5 or 4 L containers, separated as needed, and transferred to 5gal plastic containers provided by Regulatory Compliance.
c. The 2.5 or 4 L and 5 gal containers, and the storage location for the 5 gal container for say the "Acetone Waste" are all labeled the same using a label provided by EH&S.
d. In CHM 151L waste from experiment five which is a qualitative analysis of various metal cations and anions is collected in bulk as "Cation and Anion Waste".
e. CHM238L and some research labs have divided their waste streams into "Nonhalogenated Waste" and "Halogenated Waste".
The bulk method of waste collection reduces the paperwork and cost for hazardous waste collection but care must be taken not to mix incompatible used chemicals.
4. Acid Neutralization: Many laboratories generate significant amounts of used acid solutions. Used solutions that contain only acids without flammable, health, reactive, or environmental hazards can be collected and neutralized. In CHM 151L and 152L used acid solutions are collected in "Corrosive Liquids" 5 gal pails and then neutralized. These solutions should not be described as hazardous waste in the collections process.
In some labs all four systems might be used. To tell what method is being used, just look at the waste bottle. A special labeling system will be used for bulk collection. If the label lists the percent of each component, then Method 1 is being used because no log sheet would be needed. For a non-bulk bottle label not providing percentages, a log sheet would be required. The following information will describe how hazardous chemical waste is defined, classified, collected and disposed of, and overseen.
Definition of Hazardous Chemical Waste:
The Resource Conservation and Recovery Act (RCRA) mandate the proper disposal of hazardous chemical wastes. Disposal of many waste chemicals down the drain is illegal. If chemical waste has any of the following characteristics it must be collected for proper disposal (The colors noted below are used in labeling to help classify waste):
TOXIC: A poisonous substance, potentially harmful to human health, which can cause cancer or birth defects, or can contaminate, harm or kill wildlife.
FLAMMABLE: Substances that can explode, ignite, or emit toxic gases/fumes if exposed to a source of ignition.
REACTIVE: An unstable substance which can react spontaneously if exposed to heat, shock, air, or water. Reactions include fires, explosions, etc. The research director or instructor for the lab must neutralize any reactive substance before it can be accepted for disposal.
CORROSIVE: A substance could corrode storage containers, or damage human tissue upon contact. (For example, acids and bases, pH <5 or >10)
Chemical waste not fitting into the above categories may be flushed down the drain with large amounts of water. The professor must be consulted if there is uncertainty with regard to the collection of a chemical waste. The instructor or research director is responsible for the proper collection of hazardous waste.
Classification of Hazardous Chemical Waste:
All hazardous waste must be classified and stored in waste bottles. Hazardous waste must be segregated into categories to prevent dangerous mixing of incompatible chemicals. Separation of hazardous chemicals will also facilitate recycling of chemicals that is less expensive than landfilling waste. Waste must be segregated according to the four classifications mentioned above if possible. Storage codes used by chemical manufactures can also be used to classify waste. The following sub‑classifications may be used as needed:
Waste Nonhalogenated Organic Liquids: This sub‑class includes simple liquid hydrocarbons containing C, H, and O and excludes compounds containing any other elements.
Waste Halogenated Organic Liquids (or Other Elements): This sub‑class includes compounds which contain Cl, Br, F, I, N, etc. and those compounds containing complex ring structures in addition to C, H, and O. This waste cannot be easily incinerated.
Waste Organic Solids: Toxic, reactive or corrosive organic solids.
Waste Inorganic Solids: Toxic, reactive or corrosive inorganic solids.
Waste Aqueous Solutions: Solutions containing Cr, Cd, Pb, Ni, etc.
Waste Containing Mercury Compounds: Solutions containing Hg compounds.
Waste Inorganic Acid Solutions: pH < 4
Waste Inorganic Base Solutions: pH > 10
Waste Strong Oxidizing Agents: Strong oxidizing agents, such as nitric acid, perchlorate ion (ClO4‑), etc.
More waste bottles can be used to prevent mixing incompatible chemicals. Chemical waste should only be combined if you are sure the all components are compatible. A short list of incompatible chemicals is provided in an appendix of your text, "Safety in Academic Chemistry Labs". There are also several references in Room 212, which can be used to help classify hazardous waste.
Collection of Hazardous Chemical Waste:
The proper collection of hazardous waste is extremely important. Each component and its percentage must be documented. Waste must be stored safely and legally. The following guidelines must be followed in order to accomplish this. Please note that special materials such as printed labels, hazardous waste log sheets, hazardous waste tags, and colored tape needed for hazardous waste collection are available in the prep stockroom (216), stockroom (214), and the TA training Room (212) of Building 17:
Location: Waste storage areas must be away from exits and centralized in a designated location in the lab. Storage areas must be vented and kept clean.
Containers: Use only glass or sturdy‑walled plastic containers as appropriate. All previous labels must be completely removed from used containers. Waste bottles may not be stored for more than six months. The container should be chosen small enough to hold the waste generated in six months but not longer. All bottles should have caps or lids screwed loosely enough on the bottle to prevent pressure buildup. The lids should always be on except when the bottle is being used. Caps should only be screwed tightly onto bottles during transport.
Hazardous Waste Labels: Printed hazardous waste labels are available in Rooms 210, 212, 214, and 216 for bottle only and bulk collection. The labels must be filled out completely and stuck on the bottle. The chemical name and percentage of each main and hazardous component must appear on the label for collection method one. If the composition for a single waster bottle is too complex to do this, a "Hazardous Waste Log" must be kept for the bottle (see below) and a generic description of the waste is to be listed on the label.
Colored Tape: Strip(s) of colored tape above the label is used to identify hazards and classify waste. The following scheme is used:
Blue ‑ health hazard, poison, toxic
Red ‑ flammable hazard, generally organic liquids
Yellow ‑ reactivity hazard, strong oxidizers, etc.
White ‑ contact hazard, corrosive, strong acids, etc.
Orange or Green - Low hazard materials
Note: More than one color of tape may be needed for each bottle.
Hazardous Waste Logs: For any waste bottle used for several experiments or procedures, the composition may become too complex to write on a small label. In such a case, a log sheet must be kept. Keep one log sheet per waste bottle of this type. This sheet is kept on a clipboard next to the hazardous waste storage location. The log and the container must have a unique bottle number and name connecting the two. Any waste added to the bottle must be recorded on its corresponding log sheet with the name and amount of each component. Refer to the "Example Hazardous Waste Log, Label, and Tag" on the next page for an example.
Disposal of Full Bottles (Hazardous Waste Tags): When a waste bottle or 5 gal. container is full, fill out a hazardous waste tag using the label and/or the hazardous waste log sheet (summarize information from the log sheet on the tag). Clean the exterior of the bottle, securely tie or tape the tag and tape log sheet (if used) to the bottle, place the lid on the bottle, and move the bottle to the rear of the hood for pickup. An authorized student employee picks up full waste bottles from the academic labs up every week. Hazardous waste in research labs is collected by the EH&S (call to arrange for pickup). The EH&S requires that a tag be filled out before they will pickup a waste container. If a waste bottle will no longer be used, the waste should be transferred to a smaller container (if safe to do so) and the procedure above followed. The research director's or instructor's name and lab number must appear on the tag. For the waste classifications mentioned earlier, the following tape colors may be used.
|
Bottle # |
Color(s) |
Description/Composition of Waste |
Comments |
|
1 |
Red |
Nonhalogenated Organic Liquids |
(C,H,O) |
|
2 |
Red, Blue |
Halogenated Organic Liquids and/or Other Elements |
Toxic |
|
3 |
Blue* |
Organic Solids |
Toxic |
|
4 |
Blue * |
Inorganic Solids |
Toxic |
|
5 |
Blue |
Aqueous Solutions |
Toxic |
|
6 |
White |
Inorganic Acid Solutions |
Corrosive |
|
7 |
White |
Inorganic Base Solutions |
Corrosive |
|
8 |
Yellow, White* |
Strong Oxidizing Agents |
Reactive |
* NOTE: Highly reactive or corrosive waste organic and inorganic solids should have separate waste bottles. The tape color will depend on the hazardous properties of the waste.
Enforcement:
Noncompliance with this policy will create unsafe conditions. Violations should be reported to the department laboratory safety officer. Any cost incurred from disposing of incorrectly collected hazardous waste may be deducted from research funds for that professor. The Department Chair and laboratory safety officer carry the responsibility for enforcing the policy described above.
Example Generic Waste Bottles:
The next page depicts an example of a label, tag, and log that would be used to document the components of a waste stream. Additional bottles for different types of waste could also be used. Be sure to use the correct waste bottle, by checking the bottle number, tape color, and description carefully. The information should match the waste to be added to the bottle. If a log sheet is in use for the bottle, check and make sure that the components in the bottle are compatible with the chemicals about to be added.
EXAMPLE HAZARDOUS WASTE LABEL (PLACED ON BOTTLE):
Note: Red and Blue tape above label
|
EXAMPLE HAZARDOUS WASTE LOG:
|
EXAMPLE HAZARDOUS WASTE TAG (ATTACHED BEFORE PICKUP BY EH&S):
|
VII. THE USE AND MANAGEMENT OF CHEMICALS
Chemical management regarding the storage and use of chemicals in laboratories is very important. The following criteria for the management of chemical must be used when possible:
A. The use of extremely dangerous chemicals in instructional laboratories is to be avoided.
B. Chemicals will be stored away from exits and liquids must always be kept in secondary containment.
C. Flammable liquid storage is limited to 1 liter or less in glass and 1 gallon in metal safety cans. Flammable liquids, especially glass containers over 1 liter, must be stored in flammables storage cabinets whenever possible.
D. Volatile corrosive or reactive chemicals such as conc. HCl, HNO3, or Br2 should not be stored in cabinets for flammable chemicals since their fumes will react with and plug vents. Instead, they should be stored in cabinets designed for their storage.
E. Explosion proof refrigerators or refrigerators for storage of flammable liquids should be used for storing temperature sensitive or reactive flammable chemicals to slow down the decomposition of these chemicals.
a. Hazardous chemicals will only be stored in refrigerators that are explosion-proof or designed for the storage of flammable liquids.
b. No foodstuffs will be placed in a refrigerator containing chemicals.
F. Other nonvolatile inorganic and organic chemicals (solids for example) may be stored on an open shelf that is fastened to the wall. These shelves should have lips to prevent chemicals from sliding off during earthquakes. Chemical storage codes found in vendor catalogs will be used to segregate chemical storage areas.
G. Chemicals with limited shelf life must be disposed of before they become a safety hazard.
H. Current Stockroom Chemical Checkout Policy:
a. Students may not checkout chemicals without a completed requisition form signed by faculty or full time staff.
b. Students may also check out chemicals if they have been pre‑approved to do so by their research advisor, professor, or supervisor who must fill out a "Stockroom Authorization Form" (see Appendix 5). The full-time faculty or staff member who signs the requisition form or the authorization form becomes responsible for the chemical checked out and for the proper use of that chemical.
c. Material safety data sheets must be consulted prior to the checkout of any dangerous chemicals (hazard code containing a "3 or 4"). The requisition form used to check out the chemical will be stamped and signed for any 3-4 rated chemicals to indicate that the user has read the MSDS. Students cannot check out extremely dangerous chemicals.
d. The quantities of reactive chemicals purchased or checked out will be limited whenever possible. Reactive chemicals with a limited shelf life must be purchased in amounts that they are used up before their storage life expires.
e. All chemicals leaving the stockroom should have the date received on the bottle. The user should write the date the bottle is opened on the label.
I. When a research project is conclude, the work area(s) must be cleaned. Any chemical waste should also be disposed of using procedures previously described. Any left over reagents or samples must be disposed of or properly labeled if they are to be used in future research. Research students must complete these tasks before a grade can be assigned for research credit.
J. Reactive chemicals with limited shelf life should be monitored as needed (at least once per year) and disposed of by the professor responsible for that laboratory area before they become unsafe. Special attention needs to be focused on chemicals forming peroxides or undergoing polymerization when stored for extended periods (3-12 months). Many types of compounds are capable of forming peroxides and hydroperoxides. In general any organic structure having a secondary or tertiary hydrogen and an electron rich group such as oxygen, amine, vinylene, etc. attached to the same carbon might be able to form peroxides. Ethers, aldehydes, alkenes, ketones, acetals, vinylacetylenes, vinyl monomers, and aromatic hydrocarbon with benzylic hydrogens are all types of compounds known to form peroxides. The following table provides suggested storage times for some peroxidizable compounds:
|
Discard After Three Months: |
|
|
divinyl acetylene |
sodium amide |
|
isopropyl ether |
vinylidene chloride |
|
potassium metal |
|
|
Discard After One Year: |
|
|
acetal |
ethylene glycol dimethyl ether (glyme) |
|
cumene |
etrahydronaphthalene (tetralin) |
|
cyclohexene |
methylcyclopentane |
|
decalin (decahydronaphthalene) |
methyl acetylene |
|
diacetylene |
methyl isobutyl ketone |
|
dicyclopentadiene |
tetrahydrofuran (THF) |
|
diethyl ether |
vinyl ethers |
|
dioxane |
|
|
Discard After One Year (Peroxide Initiation of Polymerization): |
|
|
acrylonitrile |
styrene |
|
acrylic acid |
tetrafluoroethylene |
|
butadiene |
vinyl acetate |
|
chloroprene |
vinyl acetylene |
|
chlorotrifluoroethylene |
vinyl chloride |
|
methyl methacrylate |
vinyl pyridine |
NOTE: These compounds should be disposed of after the recommended shelf-life has passed.
Chemicals having a limited shelf life will be purchased in small quantities, which will be used before their shelf-life expires. For example, isopropyl ether, divinyl acetylene, potassium metal, and sodium amide will be purchased in quantities to be used up in 3 months or less. Shelf life for some of these chemicals and other chemicals may be extended by the addition of inhibitors or other chemicals. Picric acid, for example, can be safety stored for many years as long as it is kept moist. For chemicals such as this, a tag must be attached to the bottle to record a history of treatment (with dates) such as when water was last added to picric acid. The faculty or staff person purchasing the chemical and the stockroom manager are responsible for ensuring this policy is followed.
K. Special hazards such as carcinogens, reproductive toxins (teratogens, mutagens), highly toxic compounds, and sensitizers must be used in wall unit fume hoods. Extreme hazards must have special use documentation provided to the department safety committee by the laboratory director.
L. Some chemicals such as drug precursors have restricted distribution. The faculty person who needs any of them must come to the stockroom and obtain them in person. Under no circumstances will any of these chemicals be issued to anyone other than an identified faculty. That faculty person shall sign the requisition form, accepting full responsibility for legitimate use of the chemical. The stockroom manager maintains a list of these chemicals.
VIII. FUME HOODS
The building is equipped with state-of-the-art low flow fume hoods. You need to know how use them correctly. First of all, every hood is equipped with a flow sensor and alarm. If the flow is too slow or too fast, they may not be able to trap and remove fumes and an alarm will go off. Notify your TA or instructor immediately if this is the case. The fume hood has two modes, standby or sleep mode and normal mode. When the sash (front window of the hood) is all the way down or closed, the hood will be in standby mode and the flow rate will be about half of normal mode to conserve energy. If you pull the sash up about an inch or less, you will hear a click and the hood flow will ramp up to normal mode providing you with the best protection. The sash has sliding windows that can be used to protect you and provide access to work inside the hood with the sash closed. The hood may not function correctly with the sash all the way up. Never store chemicals or equipment in a fume hood, because this can degrade fume hood performance. Keeping this information in mind, follow these rules for safe fume hood use:
1. Never put your head inside a fume hood.
2. Keep the sash down except when setting up an apparatus.
3. Before using pull sash up just enough to ramp up to normal mode (<1”).
4. Use sliding windows to access work in hood. When doing dangerous work keep a window in front of you for protection and reach around it to work.
5. As a general rule you should work at least 6 inches inside a hood from the hood sash.
6. Notify the TA or instructor immediately if an alarm comes on.
7. Clean up any spills immediately. Get the TA’s help if the spill may be dangerous.
8. When done working in a hood, wipe down with a damp sponge, push the sash all the way down, close the sash windows, and turn off the light.
9. Never store chemicals and equipment in the hood. Instead use your bin or locker for your solutions. The storage cabinets under or to the side of the hood may also be used to store solutions. It is deemed acceptable to leave ring stands and rubber tubing for condensers in hood.
IX. CLEANING GLASSWARE
Always use proper gloves to protect your hands if needed. To properly clean glassware you need to be aware of the hazards and solubility properties of the material that was used. Be sure to properly dispose of any used chemicals if they pose a hazard. You may need to dispose of any residual material in a container if it poses a significant hazard. Containers used for volatile concentrated acids such as hydrochloric, nitric, or acetic acids or bases such as concentrated ammonium hydroxide must be rinsed in a fume hood with water to remove any residual chemical.
Water soluble materials such as ones used in general chemistry labs can often just be rinsed with hot tap water several times and then once with RO water (purified water). Fill a wash bottle with RO to rinse glassware or use the RO rinse tub in you lab. Do not use the RO water tap in you lab to rinse your glassware. Wash glassware soon after you are done using it and never leave or store dirty glassware. If need be use lab soap to help clean glassware. Hard to clean glassware such as volumetric or mohr pipets may need to be cleaned with soap in a sonicator. Sometimes with less water soluble materials you will need to use a cleaner such as simple green. This works very well with labels.
When working with volatile organic chemicals, containers may need to be rinsed with acetone in a fume hood to remove the organic material before washing at a sink. Our organic chemistry labs will have at least one acetone cleaning station in a fume hood. Never allow fumes from a volatile chemical to escape into the lab. Some organic material such as vacuum grease is insoluble in both acetone and water. Use hexanes to remove this material and then acetone to remove the residue hexanes. Collect used acetone and hexanes in a waste bottle. Do not add any other materials to this type of waste bottle.
X. Handling Reagents
The liquids, solids, and solutions used in a laboratory are called reagents. You must become well acquainted with these reagents, their containers, and their proper use. The reagents are kept on a separate bench away from your work area. Some reagents must be kept in the fume hood, because they generate flammable or toxic fumes. When you need a reagent please follow these rules:
1. Be sure to use the correct reagent, especially noting the concentration. Find the reagent, check the concentration, and then carefully read the label again to be sure you have the right one. Note the hazard code and take necessary precautions.
2. Use the reagent at the reagent bench. Do not take the reagents to your work area.
3. Please conserve and take only what you need.
4. Do not contaminate the reagents. Always use a clean spatula for solids and clean glassware for liquids. Never put a pipet or pipettor into a liquid reagent. Instead pour what is needed into a clean, dry container and take it to your work area.
5. NEVER return unused reagents, liquid or solid, to the reagent bottles. Discard or share any excess.
6. Put lids back on the reagent containers snugly and put back in correct locations.
7. Clean up any reagent you spill with a wet sponge, rinse out the sponge at the sink, and then wash your hands.
8. Use great care with corrosive chemicals (strongly acidic or basic solutions). Always wear safety goggles. Rinse your hands with tap water after using corrosive chemicals, especially if you feel a burning or slimy sensation on your skin. Wear the gloves provided in the laboratory if required. Most strong acids and bases will be disposed of as a “Corrosive Liquids” in the hood as noted in experimental procedures unless the used chemical has other hazard properties.
9. Avoid using cup sinks to dispose of nonhazardous chemicals; instead use large sinks available in the lab. Be sure to follow the instructions in the experiments with regard to the disposal of chemicals.
10. Wash all glassware that you use. Often all that is needed is to rinse well with hot tap water 4 or 5 times. If the glassware is really dirty, use detergent or simple green, then rinse hot tap water. Rinse all glassware with RO water (use RO rinse tube if one is available in your lab). Test tubes, pipets, buret, and volumetric flask should also be rinsed with a small amount of fresh RO water before storage. Fill your plastic wash bottle with RO water for doing this. You do not need to dry the inside of glassware. Never store dirty glassware!.
11. Hot objects can damage the lab bench surface. Never put hot objects on the bench top, instead place hot objects on the ring stand base or white hot pads provided.
12. Flammable or volatile chemicals must always be used in a fume hood. Place stoppers on containers when transporting them outside fume hoods. Rinse out glassware used to work with these chemicals in the waste hood before washing them in the sink.
13. At the end of every lab period you must clean your workstation bench space, hood space, and any area you used by wiping it down with a clean, damp sponge. Rinse out and wring out the sponge when you are done. Your workstation drawer must be neat and complete with clean equipment. Your locker must have no extra equipment.
XI. RADIATION SAFETY
There are two key sources of ionizing radiation used on campus: instruments using X-rays and radioisotopes. Radioisotope safety is the responsibility of the EH&S and University Radioisotope Safety Committee. Users of X‑ray generating instruments are monitored. All users must be trained in the proper use of the instruments or radioisotopes and made aware of possible hazards. Training will be conducted by the EH&S. Film badges (ring) for monitoring personal exposure must be worn when using the X‑ray units or certain types of higher energy radioisotopes (see ORC). In addition persons who have pacemakers should be aware of possible dangerous effects of magnetic fields generating FT-NMRs. Lasers are another radiation hazard requiring special training and eye protection.
XII. LABORATORY HAZARDS AND SAFETY INFORMATION
The following outline highlights important safety information:
A. Chemical Hazards:
a. Acute ‑ HCN, hydrogen sulfide, etc.
b. Chronic ‑ Hg, Pb, Cr, Cd, asbestos, others
c. Concentration Factor
d. Use Common Sense
e. Take time to get informed (Chemical literature on safety)
B. Physical Hazards:
C. Reaction Hazards:
a. Reactivity Hazards – Possible Violent Chemical Reactions
i. Peroxide Formers
ii. Oxidizing/Reducing Agents
iii. Special Cases - Picric Acid, Perchloric Acid, etc.
b. Flammable Hazards
c. Solvents
d. Combustible Liquid – flash point 100°F (38°C) to 200°F (93°C)
e. Flammable Liquid – flash point less than 100°F (38°C)
f. Water reactive chemicals
g. Air reactive chemicals – pyrophoric: ignite 5 min. contact with air
D. Health or Toxic Hazards:
a. Factors – Dose, Length and Route of Exposure, Sex, Age, Lifestyle, Sensitization, Allergic Factors, Genetic Disposition, Health
b. Acute Effect – has immediate toxic effects (toxic, corrosive, irritant, etc.)
i. Quantitate using:
· LD50 – Lethal Dose which kills 50% of orally treated animals
· LDLO – Lowest lethal dose (not in air) reported to cause death
· LC50 – Lethal Concentration which kills 50% of treated animals via inhalation on one dose
· LCLO – Lethal Concentration Low or the lowest concentration in air reported to cause death
· STEL – Short Term Exposure Limit (15 min.)
· IDLH – Immediately Dangerous To Life or Health over a 30 min. period before irreversible damage takes
· TLV-C – Threshold Limit Value Ceiling, Never exceed this concentration(air)
· Ceiling Levels (C) – Maximum level of limited exposure
· TLV-S – Threshold limit value for skin (S)
c. Terms:
· Irritant – have an irritant effect on skin, eyes, respiratory, etc.
· Lachrymator – burning effect on eyes, skin or respiratory tract
· Allergens – cause immunologic response (sensitization)
· Substances in combination – when the symptoms of exposure of each chemical are increased by each other (synergistic or potentiating effect)
· Poison - a substance that adversely affects one's health by causing injury, illness, or death.
d. Chronic Effect – may take days, months, or years for toxic effects to be noticed (dermatitis, bronchitis, cancer, liver damage, etc.)
i. Quantitate using:
· TWA – Time Weighted Average over a specified time period (way to calculate exposure, often combined with exposure limit)
· TLV – Threshold Limit Value based on 8 hour TWA
· PEL – Permissible Exposure Limit based on 8 hour TWA
· REL – Recommended Exposure Limit based up to 10 hours, 40 week TWA
· OEL – Occupational Exposure Limit based on 8 hour TWA
· Action Level – TWA exposure exceeding OSHA limit which initiates activities such as exposure or medical monitoring
· TCLO – Toxic Concentration Low or lowest air concentration known to have toxic effect or cause tumors, etc. in humans or animals
· TDLO – Toxic Dose Low or lowest dose (not in air) known to have toxic effect or cause tumors, etc. in humans or animals
e. Types of chronic hazards:
· Carcinogen – Substances which are suspected or known to cause cancer
· Mutagen – Chemical that can cause genetic alterations
· Teratogen – Can cause physical defects in fetus or embryo
· Cumulative poisons – Poisons that build up in the body
f. Routes of Entry:
i. Inhalation (most dangerous, hard to control)
ii. Ingestion (hands & adsorption in food or drinks, pipetting)
iii. Skin - S (dissolve oils, absorb through skin & can carry stuff along), Eyes worst case (fumes or splash)
iv. Percutaneous injection through the skin
g. Common Types of Health Hazards:
i. Heavy Metals (liver, kidney, neurotoxic)
ii. Chlorinated Aliphatic Hydrocarbons (skin, liver, kidney, nervous system)
iii. Aromatic Hydrocarbon (liver, kidney, respiratory and nervous system, bone marrow)
iv. Chlorinated Aromatic Hydrocarbons (liver, kidney, neurotoxic)
v. Aromatic Amines (cyanosis due to methemoglobinemia, anemia)
vi. Halogens (pulmonary, skin, eyes, neurotoxic, etc.)
vii. Phosphorus and its compounds (fire, explosion, etc.)
E. Contact or Corrosive Hazards:
a. Strong Acids (HF, HCl, HNO3, H2SO4, etc.)
b. HF presents very serious special hazards because of the fluoride ion
c. Strong Bases (NaOH, KOH, NH3, NH4OH, etc.)
d. Vapors (HCl, HNO3, NO2, NH3, NH4OH, etc.)
e. Dehydrating Agents (H2SO4, CaO, etc.)
f. Oxidizing & Reducing Agents
F. Radiation (Ionizing and Nonionizing):
G. Safety Gear:
a. Gloves (types)
b. Goggles, Face shield, explosion shield
c. First aid Kit
d. Chemical and Hg spill kits
e. Clothes such as lab aprons, lab coats, etc.
f. Dust, Masks, Respirators, fume hoods, etc.
H. Summary:
a. Be informed – read MSDS, etc. before using new chemicals.
b. Be alert! Doing Chemistry is like driving a car.
c. Use common sense
i. Be knowledgeable of chemical before use.
ii. Double & triple check labels for name and concentration and understand procedures ‑ label carefully.
iii. Use less. Be aware of concentration and temperature.
d. Play the "what if" game ‑ know location of extinguishers, safety showers, & exits. Watch for hazards
e. Don't work alone.
f. Keep work area neat and organized and wash hands after using hazardous chemicals or when done with lab work.
g. Wear safety goggles.
XIII. WHAT TO DO IN CASE OF AN ACCIDENT OR SAFETY PROBLEM
A. During your first lab period, locate the position of the fire extinguisher(s), eyewashes, safety shower, first aid kit, spill kit, phone, fire alarm pull stations, exits, showers, AED, and any other safety equipment in both the lab and hallway outside the lab.
B. In all cases of accident or injury, notify the TA, the instructor, or lab director.
C. FOR ANY SERIOUS FIRE OR INJURY: Call the POLICE DEPARTMENT (33000) from any campus phone. Security is in the best position to summon fire or ambulance service. Call the Flagstaff Fire Department (8-774-1414) or dial 8-911 if campus security cannot be reached. Use the FIRE ALARM PULL STATIONS (red box by every stairwell entrance) to clear the building of personnel. THE LOCAL FIRE ALARM IN THE LAB SCIENCE FACILITY WILL SUMMON HELP, BUT STILL ALWAYS CONTACT CAMPUS SECURITY FROM A SAFE LOCATION. THIS IS NOT THE CASE IN BLDG 20, SO ALWAYS CALL SECURITY.
D. IN CASE OF A SMALL FIRE: Immediately get help from your TA or instructor. Fire extinguishers are rated for ABC type fires in chemistry where A is combustible (paper, etc.), B is flammable liquids, C is electrical, and D is combustible metals. Use dry sand for D type fires or a special extinguisher rated for these fires. To use an extinguisher remember “PASS”: Pull the pin, Aim the hose, Squeeze the handle, and Sweep the base of the flames. If a person's clothing is on fire, they should immediately stop-drop-roll, use the safety shower if it is close, or smother the fire with a lab coat or fire blanket. Cover beaker fires with a watch glass or larger beaker to remove oxygen and put out the fire. Cool minor burns in cold water immediately.
E. IN CASE OF CHEMICAL CONTACT: If the area of contact is small, flush it well under the nearest water tap for 15 minutes. Eyes must be flushed immediately using the eyewash at one of the sinks or the eyewash by the safety shower, keeping the contaminated eye(s) open. In case of large areas of contact, start rinsing the person using the safety shower and remove contaminated clothing. After decontamination, the person will be taken to another shower facility if available in the building. Immediately inform the instructor or TA in any case. Buildings 17 and 88 both have shower facilities.
F. MERCURY SPILLS: Notify your teaching assistant or instructor. This hazardous material must be properly disposed of by collecting any visible mercury with a vacuum flask setup and sprinkling the contaminated area with powdered sulfur, which is then carefully swept up. In some cases a dust mask should be worn when sweeping up the sulfur. All of the materials and waste bottles for mercury cleanup are found in the mercury spill kit, which can be found in the prep stockroom, Room 216. Wear disposable gloves while cleaning up Hg spills.
G. CHEMICAL SPILLS: If there is any chemical contact, follow the instructions listed above and in the MSDS. Wear the correct gloves whenever spills are cleaned up. The way in which spills are cleaned up depends on concentration, size, hazard, volatility or particle size of the chemical(s). Large spills can be handled using sponges and a mop if all of the digits in the hazard code are 0 and 1. Small spill of highly hazardous material can be cause for evacuation of the laboratory. If there is any question about how to clean up a spill, refer to the MSDS for the chemical(s) involved. Here are some guidelines for cleanup of spills:
a. Small spills (a few drops to about a mL or gram) of low health, contact, or reactive hazard (hazard code of 0, 1, or 2) can be wiped up with a damp sponge which is then rinsed out with lots of water.
b. Moderate spill (over 1 mL or gram of somewhat hazardous material): Notify your teaching assistant or instructor. If the substance spilled is flammable, be sure all burners, hot plates, etc., are turned off. Spills involving concentrated acids can be neutralized and absorbed by covering with sodium bicarbonate. Every instructional lab should have a plastic tub of sodium bicarbonate. Spills of concentrated or hazardous chemicals can be cleaned up by first diking the spill to contain it. The spill is then absorbed with vermiculite, kitty litter (about 30 mesh) or sand and disposed of as hazardous waste. After absorbing a spill of a base such as 50% NaOH and sweeping it up, the remaining base on the surface can be neutralized using very dilute HCl. Cleanup materials are available in "Attack Pack Spill Kits" in every lab. Larger spill kits can be found in Room 216.
c. Large spills (about 100 mL or more of hazardous volatile material or any spill which presents a significant health hazard): First evacuate the lab area, close the door(s), and notify the instructor, lab manager (Room 212, 3-7051), stockroom manager (214, 3-2651), and/or department chairman (3-3008). Turn off sources of ignition such as burners as lab is evacuated. Faculty and staff will decide on how to proceed once the lab is evacuated. In some circumstances the building may have to be evacuated by pulling the fire alarm. If a threat exists outside the lab area (i.e.-in hallway) evacuate the building and call campus security from a safe location.
H. THERMAL BURNS: There are three types of thermal burns:
a. First Degree Burn: Skin is very red but no blistering - treat by immediately cooling in cold water for several minutes.
b. Second Degree Burn: A deeper burn but without open blisters - cool in cold water immediately for up to 30 minutes or until pain has subsided. If burn is over large area get medical treatment.
c. Third Degree Burn: A burn causing underlying tissue damage, open blisters, charring, and cell destruction - do not apply lotions or cool in water but get immediate medical attention.
XIV. EMERGENCY PROCEDURES
A. Assess Scene (0-10 seconds):
Quickly evaluate the situation (beaker fire vs. whole table, for example). Is scene safe? Do not put yourself in danger. How many victims? KEEP CALM!
B. Quick Response (less than 30 seconds):
· Evacuate laboratory immediately upon threat of fire, toxic fumes, explosion, etc.
· Use safety showers, eyewashes, or fire extinguishers as appropriate.
· Serious injury, chemical spill, fire, etc. ‑ instruct someone to call for help (dial 3-3000, use emergency phone in red boxes in north hallways in Building 20). Send someone to notify closest faculty or staff. Give your name, location and condition of injured person(s), and describe the problem.
· If appropriate, evacuate the building immediately by pulling fire alarm near stairwells. Fire alarms should only be pulled if there is an immediate threat to persons outside the lab or incident scene. Notify security by calling from a safe location (see G for evacuation plan).
· Give first aid only if needed and only to the extent needed. Wear gloves to protect yourself. In cases of trauma, DO NOT MOVE the victim unless they are in immediate danger. If victim is unresponsive send someone to call 3-3000 and get the AED. Check ABCs (airway-breathing-circulation) of victim and do CPR if required and trained. Follow the instructions provided by AED after it is opened. Stop bleeding using direct pressure, pressure points, and elevation. At the same time, open victim's airway if not breathing. Use CPR (only if trained) if there is no circulation. Treat for chemical contact in eyes immediately by washing in eyewash or shower first. Large areas of chemical contract dictate use of the safety shower. Cool first and second degree burns in cold water immediately to prevent additional damage. If electrical shock, pull plug carefully, trip the breaker, or knock the person away from source of shock using a non-conducting object such as a broomstick and follow steps above. In cases of poisoning give 2-4 glasses of water and induce vomiting except for strong corrosives, cyanide, hydrocarbons, or if victim is convulsing.
· If a safety shower was used, beware of pooling water creating electrical hazards in the location of the shower, adjacent rooms, or rooms on floors below the shower. Send someone to turn off breakers or shutdown and unplug instruments and cover with plastic.
· Interview victims and witnesses quickly and find out what chemical were involved. Examine scene quickly. Go get MSDS if chemicals involved were identified.
C. Manage Scene (30 seconds to five minutes, until help arrives):
· Make sure Emergency Medical Services (EMS) has been called or notified. Have someone wait by outside building to guide EMS to site of injured person(s) in the building.
· Remove spectators and interview witnesses.
· Treat for shock by having victim lay down, insulate with blanket and pad from 212, keep them warm, and monitor ABCs continuously (shock symptoms - pale, cold, moist skin, nausea, shallow breathing). Monitor victim(s) continuously and provide "tender loving care" (TLC). Wait for health professionals.
· Special emergency first aid based on MSDS.
· Treat minor injuries. (TA should notify instructor). If there is any question about how serious the injury is, have someone (TA, instructor, etc.) walk or drive the student to the Fronske Health Center. Always escort the injured person to the health center.
· Fill out an incident report form and get the names of the victims. Have the injured person(s) sign it when possible. Employees injured on the job also need to have their supervisor fill out a “Supervisor’s Report of Industrial Injury” to gain coverage under the University’s Worker’s Compensation Policy (see the lab manager for forms).
· Start spill clean up if safe to do so only with full time faculty or staff.
D. EMS Arrives: Provide EMS with information and aid when they arrive.
E. Analysis of Scene: What caused the incident?
F. Bomb Threats:
· If a bomb threat is received by telephone, keep the threatening person on line and have someone call security from another telephone, giving them the phone number from which the threat is addressed. Do not hang up. Note any background noise and any other identifying information.
· Notify the chemistry office, 125, 3-3008.
· Evacuate the building when notified by security. Have students remove personal belonging, quickly put away equipment, and lock lab lockers.
G. Evacuation Procedures:
· Alert building personnel by notifying security and have them evacuate the building. The fire alarm should only be pulled if there is an immediate threat to life.
· Instructors and laboratory assistants will evacuate their lectures and labs, making sure to close all windows, turn off or unplug electrical equipment, turn off all burners, and secure all doors.
· Faculty and staff members will check the floors on which their offices are located making sure all rooms are empty and secured. Aid any injured persons. All fire doors will be closed (including those between buildings and modules in Building 17) at the start of the floor checks. Signs will be posted on the entrance doors indicating the building is closed.
· Faculty and staff will proceed to the SW courtyard or lobby of the Wettaw Building (88) except those meeting emergency vehicles between the Chemistry and Wettaw Buildings or Chemistry and Biology Buildings.
H. Emergency Contacts:
A sign providing emergency phone number to contact will be located outside each laboratory area. Daytime numbers are posted. After normal working hours, the person reporting the problem or emergency should call Campus Security and provide them with the room number and the name of the primary contact person for the lab. Campus security will contact the building manager or appropriate faculty or staff at home using the phone list provided by the department.
XV. Environmental Monitoring
If there is reason to believe that an exposure to any chemical exceeds the permissible exposure limit, the laboratory safety officer shall monitor the laboratory for the specific chemical(s). The EH&S staff is available to assist with this monitoring. If the monitoring discloses that an employee's exposure exceeds the PEL, the laboratory safety officer shall take all steps necessary to reduce the exposure level through use of protective lab practices and engineering controls. The EH&S shall notify the affected laboratory workers, in writing, within 15 work days following receipt of monitoring results concerning the results of the monitoring and the steps to be taken to reduce employee exposure.
XVI. Medical Monitoring
The EH&S provides, through the Campus Health Center, appropriate medical attention to any employee who develops signs or symptoms associated with occupational exposure to chemicals. Medical attention shall also be provided where monitoring reveals an exposure above the PEL, or where a spill, leak or other occurrence results in the likelihood of chemical exposure. All medical attention shall be performed by, or under the direct supervision of, a licensed physician. Medical attention shall be provided by the Campus Health Center (or by the Flagstaff Medical Center) at no cost to and with no loss of pay to, employees. NAU will provide the following information to the physician: the identity of the chemicals to which the employee may have been exposed, a description of the conditions under which the exposure occurred, and a description of the employee's signs and symptoms of exposure. The physician shall provide the employee a written opinion which shall include the following: any recommendation for further medical follow-up, the results of the medical examination and any associated tests, any medical condition which may place the employee at increased risk as a result of exposure to a chemical found in the workplace, and a statement that the employee has been informed by the physician of the results of the consultation or medical condition that may require further examination or treatment. The physician's written statement shall not reveal specific findings of diagnosis unrelated to occupational exposure to chemicals. The laboratory safety officer should establish regular medical surveillance. Anyone whose work involves regular and frequent handling of toxicologically significant quantities of a chemical should consult a qualified physician to determine on an individual basis whether a regular schedule of medical surveillance is desirable.
APPENDIX 1 - FORMS FOR USE IN INDIVIDUAL LABORATORIES
Faculty, staff and students in their lab areas can use the forms on the following pages. Please make copies of these forms as needed.
|
LABORATORY SAFETY AUDIT |
page 1 of 3 |
|
|
Person Doing Safety Check: |
Date: |
|
|
Room #: |
Name(s) Faculty or Staff Using Lab: |
|
|
Names of Student Researchers Using Lab: |
||
|
Type of Research Being Done: |
||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
FUME HOODS AND VENTILATION (flow 50-300 lfp is acceptable flow rate for bld 17 & 88) |
||||||
|
Fumes/odors not present in lab |
|
|
||||
|
Hoods clean/clear of chemicals |
|
|
||||
|
Flammable cabinets: |
|
|
||||
|
Odors? |
|
|
||||
|
Air flow? |
|
|
||||
|
Clean cabinet screens if needed |
|
|
||||
|
Negative Lab Pressure (air flow into lab) |
|
|
||||
|
Test All hoods (attach spreadsheet if 2+) |
|
|
||||
|
NOTE: Test all hoods noting PC#, flows rates for sleep, normal, & wide open, & func. alarm, lights |
||||||
|
ACTION TAKEN (test results): |
||||||
|
|
||||||
|
|
|
|
||||
|
CHECK LIST (note: type is DC, Halon, CO2, H2O) |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
FIRE EXTINGUISHERS |
Type |
Date on Tag |
|
|
||
|
Extinguisher |
|
|
|
|
||
|
Extinguisher |
|
|
|
|
||
|
Easy access and mounted on wall |
|
|
||||
|
Seals Intact and Gauge in "Good" range |
|
|
||||
|
ACTION TAKEN: |
||||||
|
|
||||||
|
|
|
|
||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
COMPRESSED GAS CYLINDERS |
||||||
|
Strapped or chained down |
|
|
||||
|
Properly labeled w/ tag (full, in use, empty) |
|
|
||||
|
Types of gases present: |
|
|
||||
|
ACTION TAKEN: |
||||||
|
|
||||||
|
|
|
|
||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
SAFETY SHOWER & EYEWASH |
Date on Tag: |
|
|
|||
|
Safety Shower/Eyewash accessible |
|
|
||||
|
Test Sink Eyewashes & update tag |
|
|
||||
|
If Present Clean & Refill Eyewash bottles |
|
|
||||
|
ACTION TAKEN: |
||||||
|
|
||||||
LABORATORY SAFETY AUDIT page 2 of 3 |
||||||
|
CHECK LIST |
OK |
MUST TAKE CORRECTIVE ACTION |
||||
|
WARNING SIGNS & OTHER SIGNS PRESENT Must post missing signs in good locations: |
||||||
|
Eye protection required |
|
|
||||
|
No smoking, eating, or drinking in lab |
|
|
||||
|
Emergency numbers posted |
|
|
||||
|
Emergency procedures |
|
|
||||
|
Close Toe Shoes Required |
|
|
||||
|
Signs indication proper use refrigerator |
|
|
||||
|
Visible Right to Know Station Sign present |
|
|
||||
|
Check Right to Know Station for Up-to-date training records, SOPs, and MSDS or SDS |
||||||
|
ACTION TAKEN: |
||||||
|
|
||||||
|
|
|
|
||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
HAZARDOUS WASTE COLLECTION AND STORAGE |
||||||
|
Bottles properly labeled |
|
|
||||
|
Type(s) of waste collection used (list). OK? |
|
|
||||
|
Proper storage & secondary containment |
|
|
||||
|
Full containers have completed waste tags |
|
|
||||
|
ACTION TAKEN: |
||||||
|
|
||||||
|
|
|
|
||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
LAB ORGANIZATION |
||||||
|
Exits not blocked |
|
|
||||
|
Chemicals stored away from exits |
|
|
||||
|
Desks close to exits, not by hood fronts |
|
|
||||
|
Lab is clean, organized |
|
|
||||
|
Table tops clear of paper and other flammable material |
|
|
||||
|
No food or drinks in lab |
|
|
||||
|
Eye protection and gloves available/used |
|
|
||||
|
Spill Kit & sodium bicarbonate |
|
|
||||
|
First aid kit |
|
|
||||
|
Hand broom and pan |
|
|
||||
|
Broken glass container used properly |
|
|
||||
|
ACTION TAKEN: |
||||||
|
|
||||||
|
|
|
|
||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
CHEMICAL STORAGE |
||||||
|
Chemical containers have chemical name not just formulas |
|
|
||||
|
Hazard codes/warning on containers |
|
|
||||
|
Expiration date on unstable chemicals(tag) |
|
|
||||
|
Storage shelves safe (lip to prevent falling ) |
|
|
||||
|
No spilled mercury |
|
|
||||
|
Cleaning baths labeled (KOH and alcohol) |
|
|
||||
|
|
||||||
LABORATORY SAFETY AUDIT page 3 of 3 |
||||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
||||
|
CHEMICAL STORAGE CONTINUED |
||||||
|
Chemical in right storage areas & only compatibles together (use storage codes) |
|
|
||||
|
Chemical stored by storage codes: |
|
|
||||
|
No dust chemical bottles |
||||||
|
Flammable liquids cabinet (liters ) |
|
|
||||
|
No bottle < 1 liter stored outside cabinet |
|
|
||||
|
Corrosive liquids cabinet (liters ) |
|
|
||||
|
Liters outside cabinet: acid base |
|
|
||||
|
General chemical storage - solids ( Kg) |
|
|
||||
|
Reactives–oxidizers, etc. ( Kg &/or L) |
|
|
||||
|
Health hazards ( Kg &/or L) |
|
|
||||
|
Flammables Storage Refrigerator? Yes No |
|
|
||||
|
Other location (type: Kg &/or L) |
|
|
||||
|
Estimate Amounts of high hazard chemicals: |
||||||
|
Perchloric acid: L Hydrofluoric acid: L |
||||||
|
NOTE: List name, amount, and container date (attach list if needed) |
||||||
|
Peroxide formers: |
||||||
|
Water or Air Reactive: |
||||||
|
Highly reactive chemicals: |
||||||
|
Highly toxic chemicals: |
||||||
|
Carcinogens: |
||||||
|
Mutagens: |
||||||
|
Teratogens: |
||||||
|
ACTION TAKEN: |
||||||
|
|
||||||
|
|
||||||
|
CHECK LIST |
OK |
SUGGESTED CORRECTIVE ACTION |
|
MAINTENANCE |
||
|
No leaky water taps |
|
|
|
Flush out all troughs and drain pipes |
|
|
|
Check for leaky sinks (water on floor) |
|
|
|
No natural gas leaks |
|
|
|
Electrical (no bad cords, placement, etc.) |
|
|
|
Electrical (poor or use of lacement |
|
|
|
Other: |
||
|
ACTION TAKEN: |
||
|
|
||
Chemical
Inventory Form
RECORD OF
SAFETY INCIDENT
Date: ___________ Prepared by:__________________________________ Position :____________________
NOTE: Return completed form to Laboratory Manager, If medical care was required also fill out Incident Injury Report for students or visitors or for employees of NAU have supervisor fill out the Supervisor’s Report of Injury Form (SRI) and send to NAU Human Resources, Box 4113; and
2) Call State of Arizona, Workers’ Compensation Early Claims Reporting Service at 1-800-837-8583 prior to the end of the shift on the day of occurrence. For more details, visit: Human Resources Policy 4.06
UNSAFE SITUATION OR CONDITION
1. Location: Building #____ Room Number_____________________ Where in Room________________
2. Describe Incident or Condition____________________________________________________________
___________________________________________________________________________________________
___________________________________________________________________________________________
3. Action taken_____________________________________________________________________________
4. Name and phone # of witness(es) _________________________________________________________
___________________________________________________________________________________________
___________________________________________________________________________________________
___________________________________________________________________________________________
RECORD OF POSSIBLE STUDENT INJURIES
1. DATE: ___________ TIME: _________ SUPERVISOR’S NAME & PHONE: ____________________________
2. NAME______________________________ Student ID #____________________ Dana ID____________
3. ADDRESS_________________________________________________________________________________
________________________________________________ PHONE:__________________________________
4. AGE____________ If NAU Employee List Job_________________________________________________ (Supervisor must fill out online Workman's Compensation Form if incident on the job)
5. SEX: Male_____ Female_____
6. STATUS: Lab student_______ TA_______ Stockroom Clerk ______ Visitor ______ Researcher _______
Faculty or Staff_______ Other______________________________________________________________
7. Place of incident: Room #_____________ Lab location_________________ Lab or class#________
8. What was person doing when injured? (Be specific) ________________________________________
____________________________________________________________________________________________
____________________________________________________________________________________________
____________________________________________________________________________________________
9. How did the incident occur? (carefully describe) ___________________________________________
____________________________________________________________________________________________
____________________________________________________________________________________________
____________________________________________________________________________________________
10. Time and date of injury ___________________________________________________________________
11. Part of the body affected________________________________________________________________
12. Describe: First aid given if any_____________________________________________________________
Medical treatment if any__________________________________________________________________
13. Potential hazard for other occurrence: Yes____ No______
14. How was hazard corrected (if yes above)_________________________________________________
__________________________________________________________________________________________
SIGNATURE OF PERSON INJURED______________________________________DATE____________________
SIGNATURE OF EMPLOYEE COMPLETING FORM_________________________________________________
(Other
Victims & Injuries use a new form or the space below)
![]() Property and Liability
Insurance Services
Property and Liability
Insurance Services
INCIDENT INJURY REPORT
This form is to be used for all injury-related accidents involving university students, participants, visitors, etc. This form should be filled out by the NAU department employee familiar with the incident (e.g. professor, department staff). Return original form to Property & Liability Insurance Services, PO Box 4067.
This form is not to be used for an employee-related injury. Supervisors must report all employee-related injuries by following two methods: 1) Fill out the Supervisor’s Report of Injury Form (SRI) and send to NAU Human Resources, Box 4113; and
2) Call State of Arizona, Workers’ Compensation Early Claims Reporting Service at 1-800-837-8583 prior to the end of the shift on the day of occurrence. For more details, visit: Human Resources Policy 4.06
Police Report #:___________ Department: ________________________ Box #: ______
Contact Person: _______________________ Title:__________________ Phone:_______
(please print)
Instructions for Filing an Incident Injury Report
On-Campus Incident:
1. If the injured person requires medical assistance (ambulance service, paramedics) contact NAU Police at 3-3000.
2. Notify supervisor or program director concerning injury.
3. Notify NAU Property and Liability Insurance Services, at 3-2009.
4. Fill out the Incident Injury Report and send to NAU Property and Liability Insurance Services (NAU, PO Box 4067).
Off-Campus Incident:
1. If the injured person requires medical assistance (ambulance service, paramedics, police department), please call 911.
2. Notify supervisor or program director concerning injury.
3. Notify NAU Property and Liability Insurance Services, Insurance Officer, at (928) 523-2009.
4. Fill out the Incident Injury Report and send to NAU Property and Liability Insurance Services (NAU, PO Box 4067)
Northern Arizona University Property and Liability Insurance Services PO Box 4067 Flagstaff AZ 86011 Work Phone:(928) 523-2009 Fax #:(928) 523-1343 http://home.nau.edu/purchasing/plis
Revised 10/29/09
EMERGENCY NUMBERS (on campus)
(dial 928-523-xxxx for number below if using cell phone)
EMS - Ambulance.....................................3-3000
Police.........................................................3-3000
Fire..................................................3-3000 or 911
Poison Control............................1-800-222-1222
Doctor (Campus Health Services)..........3-2131
Hospital - FMC.......................................779-3366
Office of Regulatory Compliance...........3-7799 Director & Radiation Safety (John McGregor) …..…...3-7258
IE & Environmental Safety (Jim Biddle)..........................3-6109
Chemical Hygiene & Hazardous Waste(Matt Freyer)..3-1146
Biosafety Officer (Shelley Jones)………………………...3-7268
Facilities - Building Repair Work Orders.....3-4227
Chemistry Office.......................................3-3008
Prep Stockroom (Reagent Prep).............3-7059
Stockroom (Chemical Orders, etc.).......3-2651
Material Safety Data Sheets:
Hard Copies: Bld.17 rm. 212 or in lab binder or Google chemical name & MSDS
Sites: http://www.hazard.com/msds/
http://www.ilpi.com/msds/#General
http://www.ilpi.com/msds/ref/index.html
http://www.research.nau.edu/compliance/orc/
http://nau.edu/Research/Compliance/
STOCKROOM AUTHORIZATION FORM
FOR STUDENT RESEARCH ASSISTANTS and STUDENT TEACHING ASSISTANTS
This for is to be used to obtain hazardous and non-hazardous materials from the stockroom. Any Faculty Member who is supervising undergraduate or graduate students doing research or teaching shall complete this form.
Name of Faculty/Staff Supervisor (Please Print) _______________________________________________
|
Name of Student |
Account # |
Room # |
Glassware, etc. |
Chemicals |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lab Authorize Charge (Y/N)
I hereby authorize the above student research assistants and/or teaching assistants to obtain the designated materials from the stockroom. The authorized student will charge the materials to the appropriate account. The assistant has been informed of the appropriate account number to which materials will be charged. I accept full responsibility in the laboratory for the safe use of hazardous materials by my assistants. I further certify that the above assistants have received adequate and appropriate Safety Training.
Signature of Faculty/Staff Supervisor _____________________________________ Date _______________
NOTE: Please return completed forms to the stockroom or to the Laboratory Manager. A new form must be completed each semester. Materials will be checked out only to students listed on the above form.
NAU CHEMISTRY DEPARTMENT
CHEMICAL HYGIENE PLAN
AND SAFETY MANUAL
FALL 2013