------------------------------------------------------------------------
| Lecture Plan | |
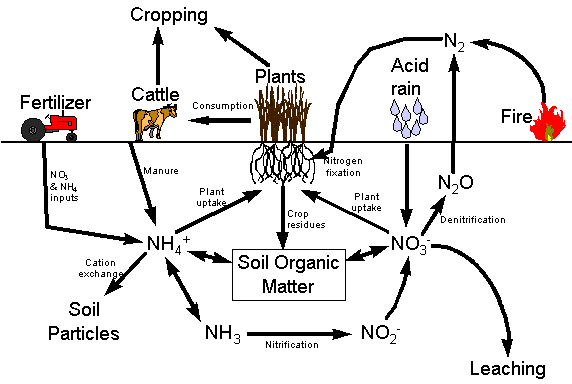
| The N Cycle | |
| Losses and Gains | |
| Important Terms | |
| N in global terms | |
| How microbes do it | |
| N balance sheets (inputs = outputs) | |

All soil N originates
from the atmosphere (not from the rocks like other nutrients)
C, P and S cycling are closely
associated with the N cycle
Sustainability is defined
as : N inputs = N outputs
(i.e. no net loss of
N from the system)
| N Gains and Losses | ||
| N Gains | ||
| N Losses | ||
Do these inputs provide enough N for sustainable agriculture in the UK?
We know cereal crops need 100-200 kg N Ha-1
Using current practices we
are not sustainable - Why ?
With future cropping practices it is also unlikely - Why ?
We don't give the soil time to recover (this loses the farmers money)
Not everybody wants to eat N2 fixing plants (i.e. beans)
N2 fixing plants need to be supercharged to increase N inputs (we haven't done this yet)
We are pushed more by economics more than sustainability (esp. by the EEC)
We need to optimize gains
and minimize losses
The problem is that we know what to do but it is not economic.
Virgil in 40 BC told us what to do: To prevent depletion
of fertility, rotate crops, leave fallow or plant legumes
![]()
| Important N cycle terms - learn these ! | |
| Biological N2 Fixation | conversion of N2 gas to organic N forms |
| Immobilisation | uptake of inorganic N and conversion to organic N |
| Mineralization | conversion of organic N to inorganic N by microbes |
| Ammonification | conversion of organic N to NH4+ |
| Nitrification | conversion of NH4+ to NO3- |
| Denitrification | conversion of NO3- to N2 |
| Fire | conversion of Organic N to N2 gas |
![]() Organic Matter N (what
is it and what is it composed of ?)
Organic Matter N (what
is it and what is it composed of ?)
| Global N statistics | N content
(x 1016 kg) |
| Atmosphere | 386 |
| Ocean | 2.3 |
| Soils | 0.02 |
| Plants and Animals | 0.05 |
| Soil-Plant-Animal Component | % in each pool |
| Plant Biomass | 3.5 |
| Animal Biomass | 0.06 |
| Litter | 0.6 |
| Soil organic matter | 90 |
| Soil Biomass (microbial & mesofauna) | 0.15 |
| Fixed NH4+ | 4.8 |
| Soluble NH4+ and NO3- | 0.3 |
| Soil Type | % of soil N as organic N |
| Upland Peat (Histosol) | 99 |
| Spruce forest (Podzol) | 90 |
| Deciduous forest (Cambisol/brown earth) | 90 |
| Tropical forest (Oxisol) | 90 |
The average residence time
for N in soil has been estimated at 175 years
![]() Nitrate is the predominant
form of N taken up by crop plants (exception is rice; NH4+)
Nitrate is the predominant
form of N taken up by crop plants (exception is rice; NH4+)
![]() The enzymes involved are nitrate
reductase and nitrite
reductase
The enzymes involved are nitrate
reductase and nitrite
reductase
![]() Ammonium is the predominant
form of N taken up by trees and microbes
Ammonium is the predominant
form of N taken up by trees and microbes
![]() N uptake is closely regulated:
supply=demand
N uptake is closely regulated:
supply=demand
![]() Most
plants do not accumulate N
Most
plants do not accumulate N
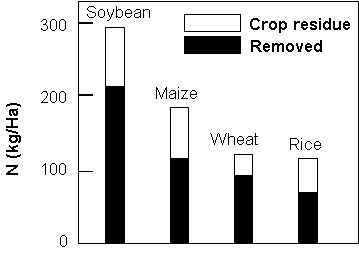
![]() 75 % of the N taken up
by a plant is removed with the crop (see below)
75 % of the N taken up
by a plant is removed with the crop (see below)
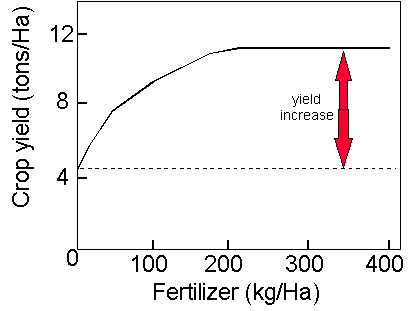
![]() Here's a typical N use efficiency curve for cereal crops. I'm sure you've seen this before but to recap, as you increase fertilizer N inputs, crop yields increase significantly until crop growth becomes limited by some other factor unrelated to N (e.g. P uptake , photosynthesis)
Here's a typical N use efficiency curve for cereal crops. I'm sure you've seen this before but to recap, as you increase fertilizer N inputs, crop yields increase significantly until crop growth becomes limited by some other factor unrelated to N (e.g. P uptake , photosynthesis)
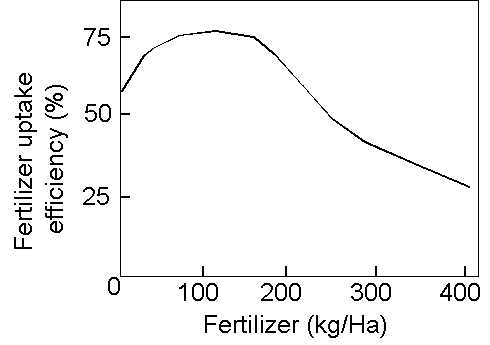
Nitrogen Losses
![]() N is the most mobile nutrient
in the soil
N is the most mobile nutrient
in the soil
![]() At least 30 % of all
fertilizer is lost
At least 30 % of all
fertilizer is lost
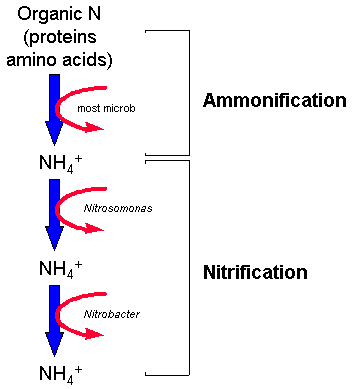
Bacterial Ammonification
and Nitrification
Ammonification
![]() When
microbes have too much N for their own requirements they excrete the excess as NH4+ into the soil. This happens mainly when the microbes are degrading crop residues with low C:N ratios e.g.
dead nodules high in N
When
microbes have too much N for their own requirements they excrete the excess as NH4+ into the soil. This happens mainly when the microbes are degrading crop residues with low C:N ratios e.g.
dead nodules high in N
![]() In high pH soils NH4+
(ion in solution) is unstable and changes to NH3 (gas)
which can be lost via volatalization
In high pH soils NH4+
(ion in solution) is unstable and changes to NH3 (gas)
which can be lost via volatalization
Nitrification
![]() This is an aerobic reaction carried
out by autotrophic bacteria which converts ammonium (NH4+) to nitrate (NO3-).
This is an aerobic reaction carried
out by autotrophic bacteria which converts ammonium (NH4+) to nitrate (NO3-).
![]() Maximal nitrification rates occur at neutral
pH and high temperatures (factors that favour the bacteria involved in this process - Nitrosomonas and Nitrobacter)
Maximal nitrification rates occur at neutral
pH and high temperatures (factors that favour the bacteria involved in this process - Nitrosomonas and Nitrobacter)
![]() The nitrification process is shown below
The nitrification process is shown below
Bacterial Denitrification
![]() Microbial reduction of
NO3- to NO2- or N2
Microbial reduction of
NO3- to NO2- or N2
e.g.
Pseudomonas
![]() Use NO3-
instead of O2 as a terminal electron acceptor
Use NO3-
instead of O2 as a terminal electron acceptor
![]() Denitrification is accelerated under anaerobic
(flooding) conditions and high fertilisers and also to a lesser extent the following:
Denitrification is accelerated under anaerobic
(flooding) conditions and high fertilisers and also to a lesser extent the following:
![]() high
temperatures
high
temperatures
![]() neutral pH
neutral pH
![]() high organic matter
high organic matter
![]() Results in environmental pollution
(destroys ozone)
Results in environmental pollution
(destroys ozone)
![]() It also contributes to global warming (nitrous oxides; minor
effect)
It also contributes to global warming (nitrous oxides; minor
effect)
Ammonia volatilisation
![]() Chemical process converting ammonium (NH4) to ammonia (NH3) which is accelerated by wind, high pH (>7) and temperature
Chemical process converting ammonium (NH4) to ammonia (NH3) which is accelerated by wind, high pH (>7) and temperature
![]() It occurs largely in arid
calcareous soils
It occurs largely in arid
calcareous soils
![]() Also occurs in soils with low exchange
capacity (e.g. sandy soils)as NH4 is not firmly held
Also occurs in soils with low exchange
capacity (e.g. sandy soils)as NH4 is not firmly held
![]() It's prevalent in soils to which farmyard manures
or urea have been added (you can smell the NH3 coming off chicken manure for example)
It's prevalent in soils to which farmyard manures
or urea have been added (you can smell the NH3 coming off chicken manure for example)
Leaching, runoff
and erosion
![]() This occurs mainly as NO3
(as it's sorbed weakly to the soil)
This occurs mainly as NO3
(as it's sorbed weakly to the soil)
![]() Accelerated under high NO3 fertilzer applications (supply> plant demand)
Accelerated under high NO3 fertilzer applications (supply> plant demand)
![]() NH4 is only leached
from sandy soils (otherwise it's firmly held on the exchange phase)
NH4 is only leached
from sandy soils (otherwise it's firmly held on the exchange phase)
![]() Leaching requires high rainfall to move
NO3 away from the rooting zone
Leaching requires high rainfall to move
NO3 away from the rooting zone
![]() Runoff - occurs when there's high rainfall, soil
compaction and no groundcover
Runoff - occurs when there's high rainfall, soil
compaction and no groundcover
![]() Erosion - wind and water carrying the soil away
Erosion - wind and water carrying the soil away
Methodology for
N research
1. Identify N-fixing micro-organisms and plants via growth in N-poor media
(you also culture predators, however)
2. Measure total N in
plant, soil or microbe by Kjeldahl-N digests (effective
but insensitive)
3. Labelled 15N
(N2, fertilizers)
4. Acetylene reduction (N2 fixation) (see later lectures)
HCHC ![]() H2C=CH2
H2C=CH2