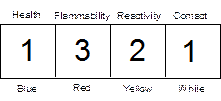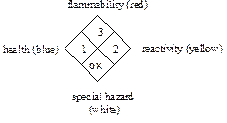LABORATORY
MANUAL
GENERAL
CHEMISTRY - CHM 152L
INTRODUCTION
CHM
152L is the laboratory course that should be taken concurrently with CHM 152,
the second semester of general chemistry. It is assumed that the key
techniques, concepts, and calculations covered in CHM 151L have been mastered. CHM
152L will explore more complex chemical concepts and problem solving. The
topics of thermodynamics, kinetics, equilibrium, spectroscopy, and redox
chemistry will be explored using various chemical reactions. Each student will
do laboratory work, data collection and record keeping in a lab notebook,
calculations, error analysis, and pre and post lab questions for each
experiment. Chemical analysis, data interpretation, and the keeping of a
laboratory notebook will be the focus of CHM 152L.
This course will be tied together
by a cooperative project conducted by a team of students. The project will
focus on the synthesis, purification, and characterization of emerald green
crystals (iron salt). The synthesis and purification of the iron salt will be
done in experiment C and then analyzed during subsequent experiments. The
analysis or characterization will be done as extensions or applications of
concepts and techniques introduced in later experiments. Student teams will
have to coordinate individual analysis work to discover the empirical formula
of the emerald green iron salt crystals and then write individual reports on
their results.
Table
of Contents: page
Course
Objectives (“I” in
the page number stands for introduction) I-2
Grading I-2
The
Laboratory Notebook I-3
Safety
in the Chemistry Laboratory I-8
Handling
Reagents and Standard Procedures I-13
Data
Recording, Significant Figures, and Error Analysis I-14
Experiments
A.
Enthalpy of Formation of MgO – Calorimetry A-1
B.
Enzyme Kinetics of Papain B-1
C.
Synthesis of a Complex Iron Salt C-1
D.
Spectrophotometric Determination of an D-1
Equilibrium
Constant
E.
Acid-Base Chemistry E-1
F.
Redox Chemistry F-1
Periodic
Table Back
Cover
COURSE
OBJECTIVES:
Laboratory manipulations and
operations including the use of a computer interfaced spectrophotometer, pH
electrode, and temperature probe.
Chemical
concepts and calculations
Including
chemical synthesis, thermodynamics, kinetics, equilibrium, acids/bases, and
redox chemistry
Computer
data collection and analysis
Construction
of graphs and plots
Record keeping with emphasis
placed on correct data collection and recording in a permanent notebook
Critical
analysis and interpretation of data
GRADING:
Grades will be assigned based on
the grading scale and point assignments listed in the course syllabus. The
point distribution is based primarily upon evaluation of the laboratory
notebook, pre-lab work and quizzes, post-lab work, reported values for unknowns,
and a final examination. In addition, two short scientific reports will be required
one for experiment B and another for the synthesis and analysis of the iron
salt.
The recording of procedures,
data, observations, and calculations in a laboratory notebook in the proper
format is a key aspect of this course. The details on how the notebook is kept
and graded are provided starting on the next page. The laboratory notebook will
be checked each lab period and it must be signed and dated after the last entry
at the end of each lab period by the TA or instructor. Error analysis will be
an important theme of post lab work done in the lab notebook. The notebook will
be formally evaluated twice during the semester.
Pre-lab work begins by reading
the experiment in the lab manual and concisely outlining key aspects of the
experimental procedure. Pre-lab questions must then be completed and a pre-lab
quiz taken before starting the experiment. Many of the questions on the pre-lab
quiz will be similar to the pre-lab questions. The post-lab work will involve
calculation and analysis of data and completing post lab work.
Students will also submit results
for unknowns that must be within given tolerance limits to receive full credit.
Points for unknowns will be assigned on how close the student’s answer is to
the correct answer. Students may repeat any unknown (using a new unknown
sample checked out from the chemistry stockroom) with the best score
contributing to their course points for that unknown.
Be aware that the CHM 152 lecture
and CHM 152L are different courses with separate grades assigned for each. Each
course must be added or dropped separately. The grade of incomplete may be
given only when a course is unfinished because of illness or other conditions
beyond the control of the student. The deadline to drop is published in the
Class Schedule.
CHM 152L
NOTEBOOK GRADING SHEET
Name______________________________
Dana ID____________ Section Letter____
Locker
#_____ Instructor_____________________ TA_________________________
Note: Each
section will be worth a certain amount of points. TAs will provide exact point
distributions. For certain experiments the point distribution may be changed or
other requirements added.
POINTS AREA
EARNED GRADED
– check indicates area is ok.
_____PTS
– General Mechanics of Notebook Entry
____ data
was recorded directly into notebook (very important)
____ chronological
order was use from older to new work (no blank areas)
____ student’s
name, class #, and section letter appears on outside of notebook
____ all pages of
notebook currently in use are numbered
____ current and
complete table of contents
____ notebook
understandable, each page and each day’s lab work started with title and date,
also each new section of work, readable
____ did not
obliterate, use pencil, or white out but “X” or line out blank space
____ all data,
calculations, and graphs are identified and have correct units
____ reasonable
significant figures were used
____ notebook
initialed and dated after each lab period’s work
_____PTS
– All data, observations, procedures, and calculation present in notebook
Experiment
Letter A B C D E F
Procedure
Outline/Changes ____ ____ ____ ____ ____ ____
Risk
Assessment ____ ____ ____ ____ ____ ____
Data
& Graphs ____ ____ ____ ____ ____ ____
Observations ____ ____ ____ ____ ____ ____
Calculations ____ ____ ____ ____ ____ ____
_____TOTAL
POINTS FOR THIS GRADING
Special
Notebook Instructions for Individual Experiments:
Experiment
A
Experimental
Data: Record all masses, volumes, and concentrations used.
Calculations:
All time-temperature data will be collected and plotted on a computer. Tape
computer generated graphs into your notebook. Record the initial and final
temperatures and the temperature change determined from each graph or trial in
your lab notebook by or on the graphs.
Experiment
B
Experimental
data: You will be doing the experimental work in groups, but each of you should
maintain a copy of the data in your notebook and tape all graphs generated into
your notebook before leaving lab. Make copies if needed.
Calculations:
All calculations are to be done individually.
Experiment
C
Student
teams formed from each lab bench will acquire the data. The observations and
data for the synthesis of the iron salt should be copied into each student's
notebook.
Experiment
D and E
Calculations:
Plot graphs on a computer. Tape all graphs into your lab notebook during the
lab period they are made. Record key information such as slope and correlation
coefficients for Exp. D and buret readings, equivalence point, etc. for Exp. E
close to or on the graphs taped into the notebook.
Experiments
F (No separate instructions)
Scientific
Integrity:
Scientific advances are based
firmly on experimental observations and depend on the accuracy and honesty of
the experimental data. The laboratory notebook must preserve the
"sanctity" of data and observations--once the measurement is taken
and recorded, it cannot be changed. "Dry-labbing” (using fake data) or
using data from past semesters is totally unacceptable because it negates the
very basis of the scientific method and is considered academic dishonesty.
Proven cases will result in serious consequences such as failing the course.
SAFETY
IN THE CHEMISTRY LABORATORY
Laboratory
safety involves the prevention of, and response to, laboratory emergencies.
Good prevention is far better than someone getting hurt. This begins with
always being aware of chemical and laboratory hazards. Hazard codes, chemical
labels, and material safety data sheets are the first sources of information
that help us prepare to work safely in a laboratory. This information can be
used to do risk assessment before starting an experiment. Certain rules
need to be followed to keep you safe and you must know what to do in case of an
emergency. Chemical waste management is another important aspect of a safe
laboratory and a key regulatory compliance issue.
Chemical Labels, Hazard Codes,
And Material Safety Data Sheets:
The first source of information
is the label on a chemical bottle. Read the label carefully before using a
chemical. A commercial chemical bottle will have extensive information on the
label such as the chemical name and formula, physical properties, purity, molar
mass, hazards, safety precautions, suggested protective equipment, and other
information. A hazard code may also be included on the label.
The chemistry department has
adopted the “Baker” hazard code classification system to inform users of
potentially hazardous chemicals. This system is designed to provide information
to people who handle chemicals in laboratories. Hazards are classified
according to four types: health (toxic), flammability (fire), reactivity
(explosive or reactive), and contact (corrosive). The intensity of the hazard
is indicated by using a number from "0" (no hazard) to "4"
(extreme hazard). This information is conveyed using either a four-colored
label found on "J.T.Baker" chemical products or as a series of four
digits. The label on chemical bottles may look like this:
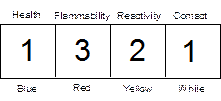
The
four-digit hazard code used in the lab manual would look like this:
1321
For
example, the code listed above for acetone indicates a slight health hazard
(1), a high flammability hazard (3), a moderate reactivity hazard (2), and a
slight contact hazard (1). Hazard codes will be listed after the chemical
inside parentheses: (1321)
The
"Baker Codes" for each
of the four hazards are defined according to the following scheme:
HEALTH (BLUE): Toxic
effects of a substance if inhaled, ingested or absorbed.
0
- No Hazard
1
- Slight hazard
2
- Moderate hazard
3
- Severe danger
4
- Deadly, life threatening
FLAMMABILITY (RED): Tendency
of a substance to burn based on flash point or the temperature a substance will
burn when exposed to a spark or flame.
0
- Will not burn
1
- Flash point above 200°F
2
- Flash point from 100-200°F
3
- Flash point from 73-100°F
4
- Flash point below 73°F
REACTIVITY (YELLOW): Potential
of a substance to react violently with air, water or other substances.
0 - Stable.
1 - Reacts
under elevated temperature or when in contact with other substances
under
other than normal working conditions.
2
- Reacts violently but will probably not explode under normal working
conditions.
3
- Reacts violently or explodes under normal working conditions when in
contact
with air, water or other substances.
4
- May react violently or detonate spontaneously under normal working
conditions.
CONTACT (WHITE): The
danger a substance presents when it comes in contact with skin, eyes, or mucous
membranes.
0
- No contact hazard to normal, healthy tissues.
1
- Slight hazard; irritant to sensitive tissues, avoid contact with eyes and
mucous
membranes.
2
- Moderate hazard; irritant to sensitive tissues, damages tissues.
3
- Severe danger; destroys tissues, including skin.
4
- Extreme danger; life threatening.
The
National Fire Protection Association (NFPA) has a hazard code system that was
adopted in 1975 to communicate hazards to emergency responders. This system
uses a label that you may be familiar with since it appears on entrances to
stores containing hazardous chemicals and on chemical containers. The NFPA may
differ from the “Baker” code since it provides information to firefighters
while the “Baker” code provides hazard information in a laboratory situation.
The codes are very similar except the white section in the NFPA code refers to
special or specific hazards of importance to firefighters such as “ox” for
oxidizing agent.
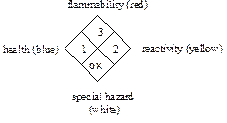
There are other hazard
communication systems that are in use or being developed. The American Coatings
Association uses the Hazardous Materials Identification System (HMIS) that is
similar to the Baker and NFPA systems but includes a code for precautions to
take for using a product or chemical. The Federal Government is in the process
of adopting the new Global Harmonized System (GHS) using a more in depth hazard
coding system that will be implemented nationally over the next few years.
The hazard codes are given only
as a guide to warn the user of probable hazards and to approximate the degree
of hazard under normal use. The user must not be lulled into a false sense of
security by a low number on the label, but must take full responsibility for
safe use of the chemicals. Avoid over-reliance on hazard codes. Refer to the
Material Safety Data Sheets (MSDS) and other safety information whenever you
are working with chemicals that are unfamiliar to you. This is especially
important when mixing chemicals. Chemicals with relatively safe hazard codes
can become dangerous when mixed with other chemicals.
The MSDS should be read to obtain
additional safety information before more a hazardous chemical is used or in
the future the Safety Data Sheet (SDS) for the new GHS. These sheets are
available for all chemicals used in the chemistry department in room 212 of
building 17 and in lab. They must not be removed from these locations. The
internet is a great resource for MSDS and general safety information. To get an
MSDS, search the internet using the chemical name and MSDS.
Risk
Assessment:
A risk assessment determines what
hazards will be encountered during an experiment or lab procedure, how to
mitigate them (precautions such as goggles or gloves), and what should be done
if something goes wrong. There may be physical or chemical hazards present that
will be discussed in the experiment write up. Chemical hazards will be
expressed using hazard codes and/or special warning stickers on bottle labels.
If you observe a 3 or 4 in the hazard code you may want to obtain more
information by referring to the material safety data sheet (MSDS) and note
hazards and how to respond to them. For every experiment you must write a risk
assessment and outline the experimental procedure before you start lab work.
How to Protect Yourself:
1. Eye Protection
MUST BE WORN IN THE LABORATORY AT ALL TIMES unless otherwise notified by the
instructor or TA. Avoid rubbing your eyes in lab unless you wash your hands
first. Use extra caution when using corrosive chemicals. Indirectly vented or
nonvented goggles are the required eye protection for this lab course. Safety
glasses or directly vented goggles are not acceptable. Do not modify or remove
the vents on goggles. Write your name and section letter on your goggles.
2. Skin protection
should be employed where appropriate; you may be required to wear long pants.
Avoid wearing shorts. The use of a lab coat or plastic apron is recommended.
Closed toed shoes must be worn at all times in the laboratory for protection
against broken glass and spilled chemicals. Open-toed shoes or sandals are not
appropriate footwear in lab areas. Disposable gloves are available for the
handling of hazardous chemicals. Always remove them before exiting the lab to
prevent contamination outside the lab. After completing lab work for the day,
wipe down your entire work area (or any area used including balances, fume
hoods, or reagent areas) with a clean damp sponge to clean up any spilled
chemicals and other material. Rinse out the sponge several times and wring it
out. Wash your hands as you exit the lab.
3. Protection from
fumes or fine powders: Never allow hazardous chemical fumes or dust to
escape into the open room; use fume hoods when necessary or specified. Be sure
to use the fume hoods correctly, following the instructions provided by your TA
or instructor. Avoid putting your head inside the fume hood and close the sash
or fume hood window whenever you are not working inside it or if it is not in
use.
4. Protection from
internal poisoning: Never "pipet by mouth", eat, drink, or smoke
in the laboratory. These activities are prohibited. Wash your hands after you
have completed lab work or leave the lab room.
5 Protection from hot
surfaces: Use the appropriate types of tongs to handle hot objects. Test
tube holders are too weak for carrying flasks.
6. Protection from fire
and explosion: Never allow flammable vapors to escape into the open room
(see No. 3). Ether is especially dangerous in this respect. Never use an open
flame while flammable liquids are being used in the room. Hot plates should be
used with care, as they are an ignition source. Flammable volatile liquids
should be used in fume hoods and stored in solvent cabinets when possible. Long
hair should be tied back to keep it away from open flames.
7. Protection from cuts:
When manipulating glassware or ceramic ware, protect your hands with a cloth
towel or leather gloves. Clean up broken glass immediately. Do not pick up
broken glass with bare hands. Use a hand broom and dustpan to dispose of glass
in the "Broken Glass Container". Do not clean up broken mercury
thermometers without help from your TA since mercury requires special disposal
procedures.
8. Protection from the
unexpected: Always read all labels noting the chemical name, formula,
concentration, and warnings (including hazard codes) carefully and double check
to make sure you have the correct chemical and concentration. Label all
chemicals with chemical name and hazard. Follow directions in the experimental
procedure exactly. Remove obstacles by keeping lockers closed, lab stools out
of aisles, and backpacks and coats stored on coat rack. For unassigned lab
work, you must have the approval of the instructor. Carefully follow hazardous
waste disposal instructions given later.
9. Safety Violations:
Any student who does not follow the above guidelines will be given one warning
and will then be removed from the lab for the day for any subsequent
violations. There may also be grade deductions or permanent removal from the
lab for serious violations.
What To Do In Case Of An Accident:
1. During your first lab
period, locate the position of the fire extinguishers, eyewashes, safety
shower, first aid kit, phone, fire alarm pull stations, exits, hallway showers,
and any other safety equipment.
2. In all cases of
accident or injury, notify the TA and the instructor.
3. For any serious fire or
injury: Call the POLICE DEPARTMENT (33000) from any campus phone or 523-3000
from a cell phone. Security is in the best position to summon fire or ambulance
service. Call the Flagstaff Fire Department (8-774-1414) or dial 8-911 if
Security cannot be reached. Use the FIRE ALARM PULL STAIONS (red box by every
stairwell entrance) to clear the building of personnel. THE LOCAL FIRE ALARM IN
THE LAB BUILDING WILL SUMMON HELP BUT STILL ALWAYS CONTACT CAMPUS SECURITY FROM
A SAFE LOCATION. Students should stay with their lab TA as the building is
evacuated if it is safe to do so.
4. In case of a small
fire: Immediately get help from your TA or instructor. Fire extinguishers are
rated for ABC type fires in chemistry where A is combustible (paper, etc.), B
is flammable liquids, C is electrical, and D is combustible metals. Use dry
sand for D type fires or a special extinguisher rated for these fires. To use
an extinguisher remember “PASS”: Pull the pin, Aim the hose, Squeeze
the handle, and Sweep the base of the flames. If a person's clothing is
on fire, they should immediately stop-drop-roll, use the safety shower if it is
close, or smother the fire with a lab coat or fire blanket. Cover beaker fires
with a watch glass or larger beaker to remove oxygen and put out the fire. Cool
minor burns in cold water immediately.
5. In case of chemical
contact: If the area of contact is small, flush it well under the nearest water
tap for 15 minutes. Eyes must be flushed immediately using the eyewash at one
of the sinks or the eyewash by the safety shower keeping the contaminated
eye(s) open. In case of large areas of contact, start rinsing the person using
the safety shower and remove contaminated clothing. After decontamination, the
person will be taken to a shower room by the prep stockroom where rinsing will
continue for at least 15 minutes or until EMS arrives if called. Immediately
inform the instructor or TA in any case.
6. In case of mercury
spillage: To dispose of this hazardous material properly, notify your TA and he
or she will collect the mercury using a special spill kit.
7. Chemical spill: If only
a few drop of chemical are spilled, immediately clean up the material with a
damp sponge and rinse out the sponge well at a sink and wipe down the area a
second time with the rinsed out sponge. In case of a larger chemical spill
immediately notify your TA and ask for help. Sodium bicarbonate (baking soda)
can be used to neutralize acid spills. If the substance spilled is flammable,
turn off all burners, hot plates, or electrical devices and get help from your
TA. For large spills notify the instructor, the laboratory manager, or the
stockroom manager. Clean-up materials are available in the lab or stockroom.
Hazardous
Waste Disposal:
The
Resource Conservation and Recovery Act (RCRA) mandates the proper disposal of
hazardous waste. Disposal of many waste chemicals by putting them down the sink
is now illegal. Regardless of regulations, the proper management of hazardous
waste is of particular importance to the people of Arizona where the
contamination of groundwater by hazardous waste could have grave consequences.
Please carefully follow the instructions below to protect our groundwater and
keep your lab safe. Hazardous waste is
determined by four properties:
TOXIC: A poisonous substance, potentially harmful to
human health, can cause cancer or birth defects, or can contaminate, harm or
kill wildlife.
FLAMMABLE: Substances, which can explode, ignite, or
emit toxic gases or fumes if exposed to a source of ignition.
REACTIVE: An unstable substance which can react
spontaneously if exposed to heat, shock, air, or water. Reactions may include
fires or explosions. The research director or instructor for the lab must
neutralize any reactive substance before it can be accepted for disposal.
CORROSIVE: A substance that could corrode storage
containers or damage human tissue upon contact. (For example, acids and bases,
pH <4 or >10)
Chemical
waste that does not fit into the above categories may be flushed down the drain
with large amounts of water. The instructor or TA must be consulted if there is
uncertainty with regard to the collection of a chemical waste.
All waste bottles are labeled and
color-coded with tape. The label will include an experiment number and a
hazardous waste description that will help you decide which bottles to put your
waste into. Find the correct waste bottle for your experiment number and for
the type of chemical waste you have; make sure the description of the
composition fits the waste you are adding to the bottle. Using the wrong waste
bottle could create a safety hazard and will be treated as a safety violation. The
following table should help. Some nonhazardous chemical waste from experiments
you do may be put down the drain. Avoid using cup sinks or water troughs to
dispose of chemicals, instead use large sinks in the lab.
Class Exp. Colors Description
of Waste Comment
152L C red Waste
Acetone Trace potassium oxalate
152L A,D,E,F
white Corrosive Liquids Neutralize
152L C,D,E,F blue Iron
Salt Crystals Waste Dehydrated or Extra
Acidic
or basic used chemicals (pH <4 or >10) will be disposed of by
neutralization in a fume hood. Waste bottles are also color-coded using the
following scheme:
Blue - health
hazard, poison
Red - flammable
hazard, organic liquid
Yellow - reactivity
hazard (strong oxidizers, etc.)
White - contact
hazard, corrosive
Green - low
hazard materials with hazard codes of 1 or less
HANDLING REAGENTS AND STANDARD PROCEDURES:
The liquids, solids, and
solutions used in a laboratory are called reagents. You must become well
acquainted with these reagents, their containers, and their proper use. The
reagents are kept on a separate bench away from your work area. Some reagents,
such as concentrated NH3 must
be kept in the fume hood because it generates toxic fumes. The reagents are
grouped according to experiment, starting with Experiment A and ending with
Experiment F. When you need a reagent please follow these rules:
1. Be sure to use the
correct reagent, especially noting the concentration. Find the reagent, check
the concentration, and then carefully read the label again to be sure you have
the right one. Note the hazard code and take necessary precautions.
2. Use the reagent at the
reagent bench. Do not take the reagent container to your work area. Any
chemicals you store should be labeled with the chemical name and hazard.
3. Please conserve and
take only what you need.
4. Do not contaminate the
reagents. Always use a clean spatula for solids and clean glassware for
liquids. Never put a pipet or pipettor into a liquid reagent, instead pour what
is needed into a clean, dry container and take it to your work area.
5. NEVER return unused
reagents, liquid or solid, to the reagent bottles. Discard or share any excess.
6. Put lids back on the reagent
containers snugly and put back in correct locations.
7. Clean up any reagent
you spill with a wet sponge, rinse out the sponge at the sink, and then wash
your hands.
8. Use great care with
corrosive chemicals (strongly acidic or basic solutions). Always wear safety
goggles! Rinse your hands with tap water after using corrosive chemicals,
especially if you feel a burning or slimy sensation on your skin. Wear the
gloves provided in the laboratory if called for. Most strong acids and bases
will be disposed of as in the “Corrosive Liquids” bucket in the fume hood as
noted in experimental procedures unless the used chemical has other hazardous
properties.
9. Avoid using cup sinks
to dispose of nonhazardous chemicals, instead use large sinks available in the
lab. Be sure to follow the instructions in the experiments with regard to the
disposal of chemicals.
10. Wash all glassware that
you use. Often all that is needed is to rinse well with hot tap water 4 or 5
times. If the glassware is really dirty use detergent or simple green, then
rinse with hot tap water. Rinse all glassware with RO water (use RO rinse tube
if one is available in your lab). Any test tubes, pipets, buret,
and volumetric flasks should also be rinsed with a small amount
of fresh RO water before storage. Fill your plastic wash bottle with RO water
for doing this. You do not need to dry the inside of glassware. Never store
dirty glassware!
11. Hot objects can damage
the lab bench surface. Never put hot objects on the bench top, instead place hot
objects on the ring stand base or white hot pads provided. Hot crucibles should
not be placed on the pads.
12. At the end of every lab
period you must clean your workstation bench space and any area you used by
wiping it down with a clean, damp sponge. Rinse out and wring out the sponge
when you are done. Your workstation drawer must be neat and complete with clean
glassware and equipment. Your locker bin must have no extra equipment.
DATA RECORDING, SIGNIFICANT
FIGURES, AND ERROR ANALYSIS
Recording Experimental Data Using
Correct Significant Figures:
It is important to
take data and report answers such that both the one doing the experiment and
the reader of the reported results know how precise the results are. The
simplest, way of expressing this precision is by using the concept of
significant figures where a significant figure is any digit that
contributes to the accuracy of an experimentally measured number or to a
number calculated from experimentally measured numbers. Please refer to the
chemistry lecture textbook for a discussion of the use of significant figures.
In this laboratory
course mass, volume, time, and temperature are experimentally measured and used
to calculate density, concentration, percent by mass, and other values of
interest. In CHM152L mass in grams (g) is measured using a top loading
electronic balance with a precision of ±0.001g or with an analytical balance if
greater precision (±0.0001g) is needed. All mass measurement taken on a top
loading electronic balance should be recorded to ±0.001g even though the last
digit may vary somewhat. For example if the mass of an object on a balance
reads 25.001, 25.000, 24.999 and moves between these values, 25.000 should be
recorded. Recording 25, 25.0, or 25.00 would be wrong since these would not
communicate the true precision of the number. If values on the balance read
25.000, 25.001, and 25.002 then 25.001g should be recorded.
Time in seconds (s) is measured
using a computer. Temperature will be measured using an alcohol thermometer
that can be read to a precision of ±0.2°C that is used to calibrate the
temperature probe connected to the computer. You need to estimate the tenths of
a degree.
Measuring volume is a tradeoff
between speed and the precision of the measurement and requires skill in
choosing the right glassware for the task. Recognizing when to make an accurate
measurement and when to be satisfied with an approximate measurement can save
much time. Frequently, the written directions will give clues to the needed
precision by using the words "approximately" or "about"
when the precision is not important and "exactly" or
"precisely" when the precision is important. Another clue
would be the number of significant figures used to write a number. For example
if a procedure says transfer 5.00 mL this must be done with great precision and
a 5 mL pipettor or volumetric pipet should be used, but instructions that say
transfer about 5 mL or 5.0 mL would indicate a measurement requiring much less
precision so that it can be done faster using a graduated cylinder. A larger
number of significant figures can and should be carried when you are using a
volumetric pipet or flask, pipettor, or buret than when you are using a beaker,
erlenmeyer flask, or graduated cylinder. Different equipment in the laboratory
is used to achieve different levels of precision. This is shown in the table
below. It is also important to note that glassware used for accurate
measurements is calibrated at a specific temperature, which is noted on the
glassware.
When a measurement is made, the
question arises: "How many digits or figures should be recorded?" The
answer is straightforward: For a measured number record all digits, which
are known with certainty, and the last digit, which is estimated. Many of
the measurements in this course involve the estimation to the nearest one-fifth
or one-tenth of a scale marking. For example, a 25mL graduated cylinder, which
has scale markings every 0.5 mL, should be read to the nearest 0.1 mL, estimation
to the nearest one-fifth of a division. Whenever estimation between markings is
being done and the reading is "on the mark," the last digit should be
included to convey the idea of accuracy to the reader. For example, with a
buret, which has markings every 0.1 mL, a reading on the mark of 11.3 mL would
be recorded as 11.30 mL; otherwise, the reader will not know that the buret was
really read to the nearest 0.01 mL. The graduated cylinder does not always need
to be used to the precision listed below; for example, if the instruction say
“add about 25mL of water” being within about 1 mL of 25 would be ok.
Generally speaking all the
glassware in the following table is for transferring known volumes of liquid
from one container to another except for the beaker and volumetric flask. Beakers
along with Erlenmeyer flasks are generally used for conducting chemical
reactions or other lab manipulations. The volumetric flask is used for doing
precise dilutions.
Precision of Glassware
|
Equipment
|
Precision
|
Purpose of Glassware/Equipment
|
|
250 mL Beaker
|
±10 mL
|
Solution preparation, storage,
reactions
|
|
125 mL Erlenmeyer flask
|
±6 mL
|
Solution preparation, storage,
reactions
|
|
250 mL graduated cylinder
|
±1 mL
|
Volume transfer – moderate precision
|
|
25 mL graduated cylinder
|
±0.2 mL
|
Volume transfer – moderate precision
|
|
5 mL bottle top dispenser
|
±0.1 mL
|
Volume transfer – moderate precision
|
|
100 mL volumetric flask (class A)
|
±0.08 mL
|
Precise final volume for dilutions
|
|
10 mL measuring pipet (Mohr)
|
±0.05 mL
|
Volume transfer – good precision
|
|
5 mL pipettor
|
±0.025 mL
|
Volume transfer – very precise
|
|
25 mL buret
|
±0.02 mL
|
Precise volume delivery for titration
|
|
5 mL, 10 mL volumetric pipet
|
±0.01 mL
|
Volume transfer – very precise
|
Another factor to take into
account when measuring volume is the level of hazard for the chemical being
measured. Bottle top dispensers will often be used to dispense more hazardous
liquids. Pump dispensers reduce the amount of transfers from one container to
another and can be used with good precision. Be sure to familiarize yourself
with the use of each type of pump dispenser. Slow deliberate use of the
dispenser will help insure that the right volume is delivered. Sometimes
approximate small amounts of liquid are needed. In this case instructions may
indicate measuring out drops from a dropper bottle or eye dropper. One drop of
water or a dilute solution on average is about 0.05 mL. This can also be a
safer method because it does not involve pouring the liquid from one container
to another.
Calculated Values and Tracking
Uncertainty Using Significant Figures:
Recorded data is then used to
calculate some value of interest in one or more steps. You will need to know
how precise or how many significant figures an answer should have depending on
the precision of the data or calculated values used in the calculation and the
type of math operation done. If you are unsure how many significant figures to
use for calculated values it is better to use too many than too few. Using too
few significant figures will introduce rounding errors into final answers!
Reporting
Answers in Addition and Subtraction:
When experimental data have been
recorded correctly, the uncertain or estimated digit is the last digit. The
calculated sum or difference of experimental measurements must be carried out
only to the place where the first digit of uncertainty enters the calculation.
Example: Add 14.75, 1.475, and
.001475 (all of which are experimental numbers). The digits of uncertainty are
underlined.
14.75
1.475
0.001475
--------------
16.226475
Since
the answer may include only the first digit of uncertainty, it should be
rounded off to that digit and reported as 16.23. It helps to line the numbers
up by the decimal point.
Reporting
Answers in Multiplication and/or Division:
1. All measurements should be recorded to
the appropriate number of digits as discussed in the section on recording
experimental data.
2. The position of the decimal point is
ignored in counting the number of significant figures.
3. All digits except zero are always
significant.
4. Zeros may or may not be significant. Any
zero to the left of the first non-zero digit is never significant (0.0256 has 3
significant figures because neither zero is significant).
a. Any zero to the right
of the first non-zero digit is always significant if there is a decimal point
(2.5070 has 5 significant figures since both zeros are significant).
b. If there is no decimal
point, zeros to the right of non-zero digits are significant unless it is
stated otherwise (the number 25000 has 5 significant figures unless some other
precision is stated, such as 25000 ± 100). Numbers with "trailing"
zeros (zeros to the right of all other digits) should be written in standard
exponential form to remove questions (2.50 x 104 has 3 significant
figures; 2.5000 x 104 has 5 significant figures).
In
multiplication and/or division, the answer should be reported to the same
number of significant figures as the value in the computation with the least
number of significant figures. Example: Find the answer to the following
multiplication/division problem to the correct number of significant figures.
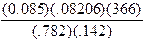
0.085
has 2 significant figures; 0.08206 has 4; 366 has 3; and 0.782 has 3. A
calculator shows the answer to be 22.989865, so the answer should be reported
as 23.
Interpretation
of Data:
Significant
figures are excellent to express the precision of raw data but not always so
good to express the precision of calculated values. As a general rule in
this laboratory course you should always use at least four significant figures
for calculated values to avoid rounding errors. Once the final answer is
calculated, it can be expressed using correct significant figures. In order to
interpret how good your results are, certain terms need to be understood. You
will need to understand the following definitions.
1. Accuracy: The term
"accuracy" describes the nearness of a measurement to its accepted or
true value. In CHM 152L, the accuracy of your work becomes known when your
unknown is graded. Full credit indicates good accuracy while a low grade
indicates that your results had poor accuracy.
2. Precision: The term
"precision" describes the "reproducibility" of results. It
can be defined as the agreement between the numerical values of two or more
measurements that have been made in an identical fashion. Good precision does
not necessarily mean that a result is accurate.
3. Range: The "range" is
one of several ways of describing the precision of a series of measurements. The
range is simply the difference between the lowest (or lower) and the highest
(or higher) of the values reported. As the range becomes smaller,
the precision becomes better.
Example: Find the range of 10.06, 10.38,
10.08, and 10.12.
Range
= 10.38 – 10.06 = 0.32
4. Mean: The "mean" or
"average" is the numerical value obtained by dividing the sum of a
set of repeated measurements by the # of individual results in the set.
Example: Find the mean of 10.06,
10.38, 10.08, 10.12
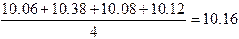
(Note that the value 10.38, which is far
greater than the other values, has a large influence on the mean, which is
larger than three out of the 4 individual values.)
5. Median: The "median" of
a set is that value about which all others are equally distributed, half being
numerically greater and half being numerically smaller. If the set has an odd
number of measurements, selection of the median may be made directly. (Example:
the median of 7.9, 8.6, 7.7, 8.0 and 7.8 is 7.9, the "middle" of the
five). For an even number, the average of the central pair
is taken as the median (Example: the median of 10.06, 10.38, 10.08, and 10.12
is 10.10--the average of the middle pair of 10.08 and 10.12). Notice in the
example that the median is not influenced much by the value 10.38, which
differs greatly from the other three values. For this reason, the median
is usually better to use in reporting results than the mean for small
data sets.
6. Error: The absolute error of an
experimental value is the difference between it and the true value. For example
if the experimental value is 30.9 and the true value is 26.5, the error would
be 30.9–26.5 or 4.4.
7. Relative percent error would be
the error divided by the true value times 100: (4.4/26.5)x100%=16.6% or 17%.
8. Relative percent range is one way
to estimate the relative percent error when we don't know what the true value
is. To calculate this value divide the range by the median value and multiply
by 100%. Using 10.06, 10.38, 10.08, and 10.12 from the previous items 3 and 5:
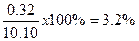
You will also be graphing data using
a program called Graphical Analysis and doing a linear fit or regression to
examine the linearity of data sets. The correlation coefficient from doing the
linear regression indicates how linear the data is where 1.0000 would indicate
perfectly linear data and smaller numbers such as 0.6000 a much poorer fit.
Your TA will provide instructions for using this program. In some cases you may
use excel or other software to graph data.