EXPERIMENT 4 - SYNTHESIS OF ISOPENTYL ACETATE
OBJECTIVE
The synthesis, purification, and characterization of an ester, isopentyl acetate (banana oil) are the objectives of this experiment.
INTRODUCTION
In this experiment an organic acid is refluxed with an alcohol to make an ester via a Fischer esterification reaction. The product will then be purified via liquid-liquid extraction and distillation. The ester, isopentyl acetate is an artificial food flavoring that tastes and smells like bananas! Obtaining 100% yield of the ester is difficult because the reactants and products are in equilibrium.
Boiling point and infrared analysis will be used to characterize the product. Please note that the above boiling points are at sea level. Boiling points measured in Flagstaff will be lower since the atmospheric pressure is less at 7,000 feet compared to sea level.
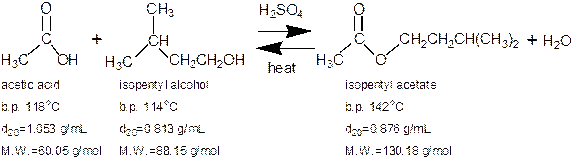
EXPERIMENTAL PROCEDURE
Prelab Preparation: Read in your lecture text about the synthesis and properties of esters. Study the lab techniques of distillation, reflux, liquid-liquid extraction, and infrared analysis in the appendix. Answer the prelab questions at the end of this experiment. Work individually on this experiment.
Glassware and Materials:
Week 1 - Reflux: distilling column, 250mL round bottom flask, ring stand, 250mL heating mantel and voltage controller, 2 clamps and clamp holders, ring support, boiling chips, ice.
Other Equipment: separatory funnel with glass stopper, pH paper, two preweighed Erlenmeyer flasks with stoppers, glass stir rod and glass wool
Week 2: Simple Distillation: 25, 50, and 250mL round bottom flasks (preweigh 25 and 50), 3-way connecting tube, West condenser, vacuum connecting tube, straight tube adaptor, thermometer, thermometer holder, two or three ring stands, 250mL heating mantel and voltage controller, 3-4 clamps and clamp holders, ring support, boiling chips, ice.
Other equipment: glass stir rod and glass wool, FT-IR
Chemicals: 20mL glacial acetic acid (2223), 4mL conc. sulfuric acid (3034), 55 mL cold RO water (0010),10mL cold brine solution-saturated NaCl in water (1001), 25mL 5% sodium bicarbonate (1011), acetone (1321), 1-2g anhydrous sodium sulfate (1121).
Cleaning Glassware: Use soap and water to clean your glassware, and to dry glassware use an oven and/or acetone. Glassware that is difficult to clean can be rinsed with hexane and then with acetone to remove any leftover hexane. The hexane will dissolve any residues of vacuum grease. Be sure to wear gloves and use a fume hood to do the rinsing and place the used hexane and acetone in the proper waste bottles labeled “Waste Acetone".
CAUTION: Exercise caution to avoid contact with concentrated sulfuric acid (3034) and glacial acetic acid (2223). They will cause serious burns if spilled on the skin. In case of contact with skin or clothes wash off immediately with excess water and use safety shower if needed. Sodium bicarbonate may be used to neutralize the acid. Clean up all spills immediately. Household acetic acid (vinegar) is typically 5% and glacial acetic acid is 99.8% pure. Wear goggles at all times! Lab coats or aprons would also be a good idea. Also be aware that acetone (1321), acetic acid (2223), isopentyl alcohol (1302), and isopentyl acetate are flammable. Keep open containers of these chemicals away from sources of ignition such as a bunsen burner flame and only work with them in a fume hood to control release of fumes into the lab. Keep containers with chemicals stoppered and rinse glassware with acetone in hood before washing at the sinks to avoid release of fumes.
Assemble Apparatus and Obtain Reactants: Assemble the reflux setup shown in the appendix on page A-2. Lightly grease the ground glass joint between the 250 mL round bottom flask and the column condenser (the bigger of the two condensers). Clamp the round bottom flask and top of the condenser so that there is enough room to raise or lower the heating mantel under the flask. Be sure to measure volume of the following reagents to the nearest 0.2 mL and wear goggles and gloves. Under the fume hood obtain 15 mL of isopentyl alcohol (also called isoamyl alcohol or 3-methyl-1-butanol) in the clean, dry 250-mL round-bottomed flask from the reflux setup. Add 20 mL of glacial acetic acid. Then, carefully add 4 mL of concentrated sulfuric acid to the contents of the flask, with swirling. Add about 4-8 boiling chips to the mixture. Boiling chips or other materials such as glass beads are always added to prevent superheating or "bumping" when doing a reflux or distillation.
Start Reflux: Get the instructor or TA to approve your apparatus before applying heat. Bring the mixture to a gentle boil with a heating mantel with the controller set on "3-5" and gradually increase. Heat the mixture under reflux (slow boil) for one hour, then remove the heating source and allow the mixture to cool to room temperature. Work on the introduction and experimental sections of your report during the reflux.
Liquid-Liquid Extraction: Isolation of Product: Filter the cooled mixture through a plug of glass wool in the glass funnel into a separatory funnel and carefully add about 55 mL of cold distilled water. Rinse the round bottom flask with about 10 mL of cold brine solution (aqueous solution saturated with sodium chloride, 34g/100mL water) and pour the rinsing through the funnel/glass wool setup into the separatory funnel. Swirl and mix the solution in the separatory funnel. Stopper the separatory funnel with a lightly greased glass stopper, shake it several times, and vent. Allow the layers to settle. Separate the lower aqueous layer from the upper organic ester layer (density of organic layer is about 0.87 g/mL). Place each layer into separate erlenmeyer flasks (preweigh flask and stopper for ester). Weigh the flask-stopper containing the organic ester layer and calculate mass of ester collected. Discard the aqueous layer in the other flask into the waste bottle labeled “Aqueous Waste”. In a wall hood place the used glass wool in waste container labeled “Waste Solids: Glass Wool, Boiling Chips, Gloves, Sodium Sulfate” and rinse the funnel and empty flask with acetone into this waste bottle also.
Neutralization of the residual acid in the ester: The crude ester in the organic layer contains a small amount of acid and other impurities. The acid can be neutralized with 5% aqueous sodium bicarbonate solution (a weak base).
H2SO4 + 2NaHCO3 → Na2SO4 + 2CO2↑ + 2H2O
BE AWARE: This neutralization can evolve significant CO2 (carbon dioxide) gas. Do not mix the product with the 5% aqueous sodium bicarbonate in the separatory funnel. The 5% sodium bicarbonate should be slowly mixed with the product in the erlenmeyer flask to prevent pressure buildup in the separatory funnel. Carefully and slowly add 25 mL of 5% sodium bicarbonate solution to the organic layer contained in the flask while gently stirring the mixture with a glass stir rod. Pour the mixture back into the separatory funnel (close the stopcock first). Rinse the flask with some cold distilled water and add to the separatory funnel. Shake the mixture gently while holding the stopper in and then allow the layers to separate. Drain about 1-3 mL of the aqueous layer from the funnel into a flask and check the pH with pH paper. Add 5 mL portions of 5% sodium bicarbonate to the separatory funnel until the pH is 5 or greater. Shake and vent the mixture in the funnel each time 5% sodium bicarbonate is added. If the pH is greater than 5 proceed on with the experiment. The aqueous layer (bottom layer) can be drained into a flask and disposed of in the waste container labeled “Aqueous Waste”. Rinse the flask with water and acetone to remove any traces of ester before washing at the sink.
Drying the Ester (removing water): Drain the organic ester solution from the separatory funnel into a clean and dry erlenmeyer flask (preweigh flask with stopper) and reweigh to get the mass of the ester after neutralization. Add 1-2 g of anhydrous sodium sulfate (a drying agent, check with your TA if you are unsure how much to use) to the solution in the flask, stopper it with a rubber stopper or cork, swirl, and let it sit for at least half an hour (good place to stop for the day).
Note: rinse all glassware that came in contact with the ester with small amounts of acetone in a hood to remove trace ester and other organics before washing them in the sink. This will help keep the lab smelling nice!
Swirling along with gravity filtration through sodium sulfate will be used to remove the water from the organic ester solution. The anhydrous sodium sulfate removes water by forming a hydrate.
Na2SO4 + 2H2O → Na2SO4•2H2O
Carefully pour the organic solution through a glass funnel with a plug of glass wool or filter paper to remove the sodium sulfate. Collect the dry organic solution in a clean, dry preweighed 250-mL round bottom flask (the flask to be heated during the distillation). Reweigh the flask to obtain the mass of the ester before distillation and note in your lab notebook.
Preparation for the Simple Distillation
Place about four drops of this ester in a clean dry vial for possible analysis. Then, add about 3-5 boiling chips to the round bottom flask. Boiling chips or some other material to prevent superheating (results in bumping) should always be added to the flask before heating it for a distillation. Some of the unreacted isoamyl alcohol in the organic solution may be removed during the previous extraction but since it is more soluble in the ester than the water, much of it will remain in the organic layer. Because isopentyl alcohol, acetic acid and water have boiling points lower than isopentyl acetate, we will separate these unwanted molecules from the isopentyl acetate by simple distillation.
Assemble a simple distillation apparatus as shown in the appendix using the smaller condenser. Use the 25 mL round bottom flask to collect the lower boiling distillate (“forerun”) and the 50 mL round bottom flask for the higher boiling fraction that should contain the pure ester. Clean, dry, and weigh these flasks so the mass of the distillate in each can be determined later. Glassware can be dried using the oven drying but not "blown dry" by the relatively "dirty" compressed air. Dry all of the clean glassware thoroughly before use and lightly grease the ground glass joints. WHY? Get the instructor or TA to check your apparatus before beginning. (Note: The larger condenser may need to be used or the apparatus tilted down toward the receiver flask if distillate collects in the condenser, see your TA).
During the distillation, we will collect the unwanted distillate called the "forerun" from 50°C to about 110°C in a 25 mL round bottom flask and save it for later analysis. If the temperature increases quickly to 110°C just collect the first 10 drops of distillate as the forerun. When the temperature begins to increase into the 110-120°C range, quickly switch the 25 mL flask for the previously weighed, 50-mL round bottom flask (or if the temperature drops and then increases to 110°C). Use ice in a beaker to cool the receiving flask. The ester you collect should be quite pure, distilling from 110 -130°C. Do not heat the distilling flask to dryness. Stop the distillation when there are a few drops of liquid left in the distilling flask.
Simple Distillation
Begin the distillation by setting the heating mantle controller on to about "50-60 volts". Increase the setting as needed up to about "70-80 volts" to maintain a moderate boil. Switch flasks as noted above. Monitor time, temperature, and estimated volume collected as the distillation progresses in your lab notebook. From this data you can obtain the boiling point of your product. When the distillation is complete, dry the outside of the flask, and weigh the product (i.e. subtract the initial weight of the empty flask from the final weight) to obtain the actual yield. Calculate the percent yield of your product, isopentyl acetate, at each step in the purification process. Remember that the percent yield is:
![]()
Obtain an infrared spectrum (IR) of your product. If you have time get an IR of the product sampled before the distillation and an IR of the forerun. Isopentyl Acetate may be disposed of in the “Waste Acetone” waste bottle.
Report Requirements:
The goal of the report for this experiment is to give you background in writing using a scientific format. To accomplish this goal, you will first read some papers out of scientific journals and then write a report on the work done in this experiment. Be sure to read the section on report writing in the introduction of this lab manual. All the work submitted should be typed.
Before you start writing, go to the periodicals section of the library and read at least one article (scientific paper) out of a recent issue of the Journal of Organic Chemistry and at least one article out of a scientific journal that is of interest to you. Make copies of the abstracts found on the first page of the papers you read. You will find the format of papers will vary somewhat from journal to journal.
As you read these articles, note the format and how the information is presented. Do not worry about understanding the content of the articles; instead focus on the format and writing style. The formal lab reports you write for this laboratory should follow this same format and writing style.
Write a paragraph summary on each article focusing on the article's format and the type of information presented in each section. Next, write a paragraph comparing the writing style of the articles you read to the style found in a personal informal letter. How do the styles differ? Attach copies of the two abstracts and three paragraphs you wrote as an appendix to your report. This part of the report should be completed and turned in to your TA on the second day of this experiment at the start of the period.
Now that you have a general idea of what a scientific report or paper looks like, you can start writing your report! The section on reports in the introduction and the grading sheet for the report at the end of this experiment will give more specific guidelines on report writing. Be sure to refer to your lecture textbook also. This report should be brief (not more than five pages of typed text). Use data to support all of your conclusions. Clearly explain your findings in a formal laboratory report including an abstract, introduction, experimental method, results, discussion of results and error analysis, and reference section.
In your report there are several key issues to be addressed. Be sure to analyze the boiling point data and identify all of the peaks in IR spectra. Use your knowledge of every chemical involved in this experiment, and their boiling points in addition to the IR data to convince the reader that pure isopentyl acetate was or was not produced. Interpret and explain all of your data in detail! Be sure to address every item on the grading sheet for this report. Read about the properties of esters and review the experimental procedure. In addition, comment on why the percent yield for this experiment is less than 100% and at which steps in the procedure you might have lost product. All lab reports must be typed and done individually. Do not write joint reports and do not copy from old reports.
STUDY GUIDE - PRELAB QUESTIONS:
These questions are designed to help you understand and be prepared for this interesting laboratory experiment! Be prepared to take a quiz covering the reading and the prelab questions before beginning this experiment.
1. Risk Assessment: What are the safety hazards and precautions for this experiment?
2. How is the glacial acetic acid and sulfuric acid in the product neutralized after reflux (show the two reactions)?
3. When doing the distillation, which chemical would be the first, second, third, and fourth to distill across assuming all the reactants and products are present, based just on boiling point?
4. What is the purpose of the reflux and how does it work (hint - see appendix)?
5. Why would you use cold instead of hot distilled water to rinse an organic liquid that is slightly water-soluble?
6. Determine the limiting reagent and theoretical yield for this experiment. What would the percent yield be if a student collected and purified 1.34 g of the ester? Show your calculations.
7. Read about infrared spectroscopy in the appendix and in your lecture text. Look at the reaction on page 4-1; what functional group is present in the reactants but not in the ester? At what wave number range in the IR spectrum does the peak appear and what will it look like?
8. What is the purpose of the sodium sulfate? Write the reaction to show how the sodium sulfate works. If sodium sulfate was not used what unwanted peak might be observed in the ester's IR spectrum?
9. What is it about the esterification reaction that makes it very difficult to obtain a 100% yield?
10. Why is the 5% sodium bicarbonate added slowly?
11. What are two other names for isopentyl alcohol?
12. What happens to the boiling point of liquids as you go from sea level to Flagstaff at 7,000 feet?
CHM 235L Report Grading Sheet - Synthesis of Isopentyl Acetate
(Staple this sheet to the front of your report)
STUDENT'S NAME_________________________SEC. LETTER___DATE______ DANA ID_____
POINTS POINTS AREA GRADED: Check indicates area OK.
EARNED POSSIBLE
____PTS 5 pts ____ Overall organization, spelling, grammar, significant figures, typed report
____PTS 15 pts – APPENDIX (turn this section in on the day you complete this experiment)
____ Copy of abstract and summary of paper from Journal of Organic Chemistry (focus on article's format/style)
____ Copy of abstract and summary of paper from another scientific journal (focus on article's format/style)
____ Comparison of writing styles of scientific papers read to a personal letter
____PTS 5 pts - ABSTRACT (paragraph which includes a summary of the objectives,
experimental, results, and conclusions)
____PTS 10 pts -INTRODUCTION
____ Brief and general background and objectives
____ Specific chemical reaction studied and how it is used to make the product
____PTS 10 pts - EXPERIMENTAL
____ Brief summary of experimental procedure
____ Briefly explain how the product was identified
____PTS 15 pts - RESULTS (data in tabular form and required calculations)
____ Mass of product at each stage in purification
____ Boiling point data
____ Properly labeled IR of pure product (also product before distillation and forerun if done).
____ Show your theoretical yield and percent yield calculations.
____ Briefly explain data presented. Do not analyze it here; just explain what the data represents
____PTS 15 pts -DISCUSSION OF RESULTS
____ Use data (IR and boiling points) in your discussion to prove you did or did not make isopentyl acetate
____ Use data (IR and boiling points) to evaluate product purity
____ Quality error analysis: You are not penalized for error but rewarded for recognizing it. Address why the yield is <100% - where was product lost?
____ Questions posed by instructor or TA
____PTS 5 pts - REFERENCES
____ Use at least 2-4 references (lab manual and lecture text could be two of them)
____ Use standard scientific format (cite in the text of paper, references at the end)
___PTS TOTAL POINTS out of 80 for the Report