EXPERIMENT 6
DETERMINING THE REACTION MECHANISM OF A CHEMICAL REACTION USING KINETICS: A LABORATORY EXERCISE
BACKGROUND
You know that the rate of a chemical reaction depends on the temperature at which the reaction takes place and the concentration(s) of the molecules or ions that must collide during the slowest step in the series of reactions that make up the reaction mechanism. (The slowest step is sometimes called the rate-determining or rate-limiting step.)
In this experimental series, you will use these principles to find out how the slowest step of a chemical reaction occurs. The reaction is a nucleophilic substitution reaction. Nucleo-means nucleus and -philic means loving. Since you know that a nucleus is positively charged, then a chemical species that loves nuclei would love positive charges. Substitution just means that you exchange or substitute one thing for another. Therefore a nucleophilic substitution reaction is one in which one molecule or ion that loves nuclei is exchanged or substituted for another molecule or ion that loves nuclei. Nucleophilic molecules have an unshared pair of electrons that can be stuck to the positive nucleus (electrons are negative and are therefore attracted strongly to positive nuclei) and nucleophilic ions are usually negatively charged anions. The overall stoichiometry of the specific reaction you will study is:
OH¯ + (CH3)3CBr ® (CH3) 3COH + Br¯
hydroxide tertiary tertiary bromide
anion butyl bromide butyl alcohol anion
The nucleophilic ions are both anions (OH¯ and Br¯), and one of them (OH¯) is substituted for the other (Br¯). The carbon kernel to which the three methyl (CH3) groups and the bromide are attached to in tertiary butyl bromide is the positive particle that the nucleophilic (OH¯) wants to become attached to.
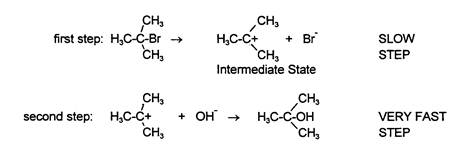
This reaction can happen in one of two ways: (1) by having (CH3) 3CBr ionize into (CH3) 3C+ and Br¯ first as the slowest or rate determining step in the reaction, followed by having the OH¯ then become attached to (CH3) 3C+,
or by having OH¯ collide with tertiary butyl bromide on the side opposite the bromide group, with hydroxide bonding to the carbon on one side at the same time that bromide is becoming unbonded on the other side,
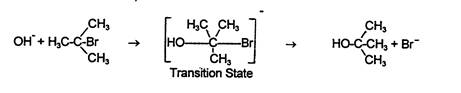
The first of these two mechanisms, the one in which the tertiary butyl bromide ionizes first and then reacts with the hydroxide, is called an SN1 reaction: S = Substitution, N = Nucleophilic, and 1 = first order. The order tells the number of reactant molecules or ions involved in the slow step. Since the slow step here is the ionization of tertiary butyl bromide, only the tertiary butyl bromide is a reactant; therefore only one molecule is involved in the slow step and the reaction is first order. The second mechanism, the one in which the negatively charged OH¯ is attracted to the partial positive charge on the carbon which releases the good leaving group, Br¯ ion, is an SN2 mechanism. A second order nucleophilic substitution is when two reactant molecules are involved in the slow step as reactants.
If the reaction occurs by way of an SN1 mechanism, then changing the tertiary butyl bromide concentration will change the rate of the reaction, because tertiary butyl bromide is involved as a reactant in the slow step. However, changing the hydroxide concentration will NOT change the rate of the reaction because hydroxide is NOT involved as a reactant in the slow step. If, however, the reaction occurs by way of an SN2 mechanism, then changing the concentration of tertiary butyl bromide will change the rate of reaction and so will changing the concentration of hydroxide, since both of them are involved as reactants in the slow step.
Experimentally, you can tell the difference between the two mechanisms by first changing the tertiary butyl bromide concentration while keeping the hydroxide concentration the same and determining whether the rate varies. Secondly, the hydroxide concentration can then be changed while keeping the tertiary butyl bromide concentration the same to see if the rate varies. If the rate changes when tertiary butyl bromide concentration is changed, either mechanism is possible since tertiary butyl bromide is involved in "the slow step" of both mechanisms. But if the rate also changes as the hydroxide concentration is changed, then only the SN2 mechanism is possible. Conversely, if the rate does NOT change with different hydroxide concentrations, then only the SN1 mechanism is possible since the hydroxide could not be involved in "the slow step" of the reaction mechanism.
A second set of experiments is used to measure the rate of reaction when keeping both tertiary butyl bromide and hydroxide concentration the same, but changing the nature of the solvent. The intermediate state for the SN1 mechanism (the middle of the reaction where the reactants have already reacted in the slow step but the final products have not yet been formed) has more charges than the transition state (shown bracketed above) for the SN2 mechanism. In fact, the SN1 intermediate state has twice as many charged particles as the SN2 transition state. Therefore, a polar solvent like water should be much better for the reaction if it goes by an SN1 mechanism than a relatively non-polar solvent like acetone. That is, if the reaction goes by an SN1 mechanism, then the reaction should be much faster in water than in acetone because it is easier to make charged particles in a polar solvent like water than it is in a non-polar solvent like acetone. On the other hand, if the reaction goes by using a SN2 mechanism, then changing solvents shouldn't make much difference in the reaction rate. This is because there is one charged particle at the start (OH¯), one charged particle in the transition state (the species drawn above in which the bromide and hydroxide are both half-attached to the carbon) and one charged particle in the end (Br¯). In summary, in an SN1 mechanism there are more charged particles in the intermediate state than in either the beginning or the ending states. In an SN2 mechanism, the number of charged particles stays the same throughout. This is why the rate of an SN1 reaction varies with the changing polarity of the solvent while the rate of an SN2 reaction does not.
Obviously, the results of these two experiments (varying the concentrations of the reactants and varying the polarity of the solvent) should agree with each other; that is, each should confirm the results of the other.
EXPERIMENTAL
PART ONE: EXPERIMENTALLY DETERMINING THE EFFECT OF REACTANT CONCENTRATION ON THE REACTION RATE.
Background:
In each run below, you will use a large excess of tertiary butyl bromide compared with hydroxide. What you will actually be measuring is the time required for all the hydroxide to be used up in the reaction. To see exactly when all the hydroxide has been used up, you'll use the acid-base indicator bromophenol blue, which is blue as long as hydroxide is present but turns yellow as soon as the hydroxide is used up causing a rapid decrease in the pH. The reaction rate will be defined as the change in tertiary butyl bromide concentration during each second of reaction. Since the reaction stoichiometry is 1 to 1, and since you are starting with a large excess of tertiary butyl bromide, its concentration change will be the same as the starting concentration of hydroxide.
Materials:
100 mL beakers, a 25 mL graduated cylinder, timer, magnetic stirrer, magnetic stir bar.
Chemicals:
45 mL acetone (1421), 170 mL 0.030 M tertiary butyl bromide (3421), distilled water, 190mL 0.0100 M NaOH (2013), 0.1% aqueous bromophenol blue indicator (1102). These chemicals will be used for Parts l and II.
Hazards:
Acetone and t-butyl bromide are flammable - keep away from open flame and use small amounts, 0.0100 M NaOH is mildly corrosive - keep out of eyes. t-butyl bromide is a carcinogen. Wash hands in of case chemical contact and after experiment.
Hazardous Waste:
Dispose of used reaction mixtures by placing in waste bottle labeled “Experiment 6 Waste (48% Acetone, 0.1% t-butyl bromide, 52% water)''. Extra 0.0100 M NaOH can be flushed down the drain. Extra acetone can be placed in "Acetone Waste'' in the hood.
Procedure – Part I
1. Students should work in pairs on this experiment and share equipment and glassware. If there is an odd number of students, one student can work alone or join a group of two. The calculations and report are done individually.
2. You will prepare the solutions listed in Table I. In trials 1-3, the concentration of NaOH is constant while the concentration of t-butyl bromide is different. For trials 1, 4, and 5, the concentration of t-butyl bromide is constant while the concentration of NaOH changes. For each trial use a graduated cylinder to transfer solutions to beaker A and B except 0.1% bromophenol blue which is dispensed with an eyedropper (solution A should be blue).
Table I
Effect of Concentration
|
Beaker A |
Beaker B |
||||
|
Trial # |
0.0100 M NaOH in water (mL) |
Distilled Water (mL) |
0.1% Bromo- phenol blue |
0.0300 M t-Butyl Bromide in Acetone (mL) |
Acetone (mL) |
|
1 |
25.0 |
0 |
6 drops |
25.0 |
0 |
|
2 |
25.0 |
0 |
6 drops |
20.0 |
5.0 |
|
3 |
25.0 |
0 |
6 drops |
15.0 |
10.0 |
|
4 |
20.0 |
5.0 |
6 drops |
25.0 |
0 |
|
5 |
15.0 |
10.0 |
6 drops |
25.0 |
0 |

3. Add a stir bar to Solution A and mix at a slow speed on a magnetic stirrer (If the solution is stirred too fast it may not change color). Quickly add all of Solution B to Solution A while simultaneously starting the timer. Stop the timer when solution changes from blue to yellow. Record and report the extent of color change. Why is this important? Try to be consistent with the timer and stop it at the same color change intensity with each run. Place the reaction mixtures in the Exp. 6 waste bottle but not the stir bar! Repeat this process for each trial. Any extra indicator can go down the drain. Record the time taken for the color change in a table form:
Trial Molarity in Mixed Time Rate
# Solution (0.0500 liters) (sec.) (mol)/(L x sec)
_____ t-Butyl Bromide NaOH ____ ___________
Calculations:
For this experiment the calculation of rate is based on the molarity of the NaOH in the final solution (resulting from the combination of the solutions in beakers A and B). This is true, because the color change is observed after all the OH is used up in the reaction and because OH is the limiting reagent. The [OH] used in the calculation is not
0.0100 M, but needs to be calculated based on the final reaction volume (volume of beakers A and B).
The initial volume of 0.0100 M NaOH is diluted to 50 mL since all the solutions have a total volume of 50.0 mL, if properly prepared. Calculate the concentrations of NaOH ([OH¯]) and t-butyl bromide in the mixed solutions knowing that the concentrations of the reagents were 0.0100 M and 0.030 M respectively (Hint: v1m1=v2m2 where v2=50 mL and v1 is the volume of NaOH or t-butyl bromide used). Put the concentrations for the mixed solution in the table mentioned above. Please note that the calculations are to be done by each student and not as partners.
To calculate rates of reaction for each trial, divide the change in concentration of tertiary butyl bromide (equals the [OH¯] as can be seen in the equation above) by the reaction time (in seconds). The change in concentration of the tertiary butyl bromide will have the same numerical value as the initial concentration of hydroxide because you have a large excess of tertiary butyl bromide and will be using up all the hydroxide.
Part I questions to be answered as you write the discussion in your report:
1. Based on your data and calculations, does changing the concentration of tertiary butyl bromide initially present in the reaction mixture cause the RATE of reaction to change? (Remember that the rate is something you have to calculate. It is not the same thing as the time required for the reaction.)
2. Again, based on your data and calculations, does changing the initial concentration of hydroxide cause the rate of reaction to change?
3. Is the rate law for the reaction studied in part 1 of this experiment R = k[(CH3)3CBr] or R= k[(CH3)3CBr][OH¯]? Be sure to explain how your data support your answer.
4. Is the reaction SN1 or SN2? Be sure to explain your reasoning and state the type of mechanism you think this reaction has.
PART TWO: EXPERIMENTALLY DETERMINING THE EFFECT OF SOLVENT POLARITY ON THE REACTION RATE
Background:
In each of the four runs below, you will be changing the percent or fraction of acetone in the reaction mixture. Since water is much more polar than acetone, the assumption is that a mixture of water and acetone will have characteristics somewhere in between the two solvents. A mixture containing more acetone than water is assumed to be less polar than a mixture containing more water than acetone. Other reaction conditions are the same as described in the background for experiment one.
Materials, Chemicals, Hazards, and Hazardous Waste: See Part I.
Procedure: The procedure is the same as that used in Part I except the solution compositions used are listed in Table II and not Table I.
Table II Effect of Solvent Polarity
|
Beaker A |
Beaker B |
||||
|
Trial # |
0.0100 M NaOH in water (mL) |
Distilled Water (mL) |
0.1% Bromo- phenol blue |
0.0300 M t-Butyl Bromide in Acetone (mL) |
Acetone (mL) |
|
6 |
20.0 |
0 |
6 drops |
15.0 |
15.0 |
|
7 |
20.0 |
5.0 |
6 drops |
15.0 |
10.0 |
|
8 |
20.0 |
10.0 |
6 drops |
15.0 |
5.0 |
|
9 |
20.0 |
15.0 |
6 drops |
15.0 |
0 |
Note that total final volume is 50.00 mL in each case. Record the time for each reaction in your lab notebook using the following table format:
Trial Time Percent
Acetone Rate
# (sec) (by total volume) mol/L-sec
7 ((15+10)/50) x100%
The total volume of acetone in the solution is the volume of acetone used plus the volume of t-butyl bromide solution since acetone makes up more than 99% of the t-butyl bromide solution. Assume 0.030 M t-Butyl bromide solution is 100% acetone when calculating percent acetone in the reaction mixture.
Calculations: Since the starting concentrations of both tertiary butyl bromide and hydroxide are the same for every reaction in this experiment, you will need to calculate their concentrations only once. Tertiary butyl bromide is present in great excess compared with hydroxide so the change in tertiary butyl bromide concentration is the same as the starting concentration of hydroxide. For this experiment, therefore, the change in tertiary butyl bromide concentration is equal to the initial concentration of hydroxide in the reaction mixture. Don't forget that the NaOH is diluted from 20 mL to a final total volume of 50mL.
To calculate the percent acetone in each reaction mixture, just divide the amount of acetone in the mixture (total volume in Beaker B) by the total volume and multiply this fraction by 100.
To calculate the rate of reaction for each of the four mixtures, divide the change in concentration of tertiary butyl bromide (hydroxide molarity in 50.0 mL volume) by the time in seconds required for the reaction from initial mixing until the color change.
Part II questions to be answered as you write the discussion of your report:
1. Based on your data and calculations, does solvent polarity affect the rate of the reaction? Be sure to give your reasoning.
2. Given your answer to question 1, above, does this experiment indicate that the reaction is SN1 or SN2? Give your reasoning.
3. Do the results of part two agree with the results of part 1?
Report Requirements:
The goal of the report for this experiment is to give you more background in writing using a scientific format. The section on reports in the introduction and the report grading sheet at the end of this experiment will give more specific guidelines for report writing. Be sure to refer to your lecture textbook also. This report should be brief (not more than five pages of typed text). Use data to support all of your conclusions. Clearly explain your findings in a formal laboratory report including an abstract, introduction, experimental methods, results, discussion of results and error analysis, and references.
All lab reports must be typed and done individually. The time in seconds is the only information that is shared. All calculations and writing must be done individually. Do not write joint reports and do not copy from old reports.
As an optional extension, you might investigate the effect of using t-butyl fluoride, chloride, or iodide instead of t-butyl bromide on reaction rate. Look at the effect of changing leaving groups (refer to your textbook and the scientific literature).
Experiment 6 Study Guide - Pre-lab Questions
1. Draw the specific chemical reaction studied in this experiment and the two mechanisms by which it takes place. Explain the differences between the two mechanisms.
2. What is the limiting reagent in this experiment?
3. What equation is used to determine the rate of the reaction? Explain.
4. What is the rate for trial 1 (see page 6-4) if the reaction mixture took 25 seconds to turn from blue to yellow after mixing the solutions in beakers A and B?
5. Why do we vary the percentages of acetone in Part 2?
6. Why is the polarity of the intermediates a factor and how do we use polarity to determine the mechanism of the reaction?
7 When will the indicator bromophenol blue turn from blue to yellow in the timed reaction? Why is this important?
8. Given these three rates, which is the fastest?
R = 1.5x10-3 (mol)/(L x sec)
R = 6.3x10-3 (mol)/(L x sec)
R = 1.0x10-2 (mol)/(L x sec)
9. What experimental parameter is varied in part I? Why is it changed?
10. Risk Assessment: What are the safety hazards and precautions for this experiment?
CHM 235L
t-BUTYL BROMIDE KINETICS REPORT GRADING SHEET
(staple this report sheet to the front of your report)
NAME__________________________ DANA ID ______SEC. LETTER___ DATE_____
POINTS POINTS AREA GRADED: Check indicates area ok.
EARNED POSSIBLE
____PTS 10 pts -Overall organization, spelling, grammar, and significant figures, typed
report
____PTS 10 pts - ABSTRACT (paragraph which includes a summary of the objectives, experimental, results, and conclusions)
____PTS 10 pts - INTRODUCTION
____ Brief and general background and objectives
____ Specific chemical reaction studied
____ Brief discussion of SN1 and SN2mechanisms and their
reactant and solvent dependence
____PTS 10 pts - EXPERIMENTAL
____ Brief summary of experimental procedure
____ Briefly explain how the procedure will determine a SN1 or SN2
mechanism
____PTS 15 pts - RESULTS (observations, data, calculations)
____ Table listing molarity of reactants, time, and rate part 1
____ Table listing time, % acetone, and rate for part 2
____ Other calculated values
____ Understandable format
____PTS 15 pts - DISCUSSION OF RESULTS
____ Interpretation of reaction mechanism part 1
____ Interpretation of reaction mechanism part 2
____ Mechanism and rate law for reaction given
____ The questions asked on pages 6-5 and 6-6 should be
answered as part of your results and discussion sections
____ Data used in discussion to support conclusions
____ Error analysis: you are rewarded for finding and explaining
error
____PTS 10 pts - REFERENCES
____ Use at least 2-4 references (lab manual, text)
____ Use standard scientific format (cite in the text of your paper)
____PTS TOTAL POINTS OUT OF 80.