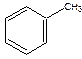Experiment 7
Thin Layer Chromatography of Analgesics
And Amino Acids
INTRODUCTION
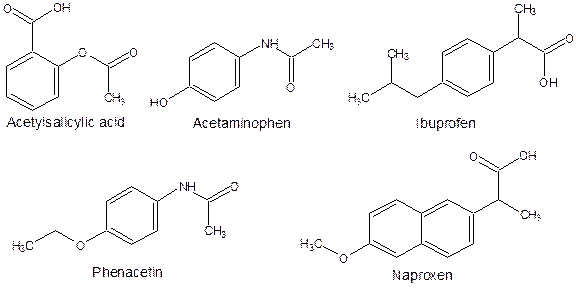
Analgesics such as acetylsalicylic acid (AA), acetaminophen (Ac), ibuprofen (I), naproxen (N), and phenacetin (P) are used for relief of pain due to headache, arthritis, etc.
Caffeine (C) is often added to various pharmaceuticals (analgesics, migraine medication, etc.) to help enhance the performance of the various drugs, due to its fatigue-reducing properties. Small doses of caffeine (50-200 mg) can increase alertness and reduce drowsiness and fatigue.[1] Caffeine is the most widely used stimulant.

Many commercial analgesics will have mixtures of the analgesics listed above along with caffeine: Aspirin (AA), Tylenol (Ac), Advil and Motrin (I), Anacin (AA,C), and Excedrin (AA, Ac,C). Thin-layer chromatography will be used to test for the presence of analgesics in a commercial product of unknown origin.
Amino acids are the building blocks of proteins. Proteins are essential to life as we know it. A protein consists of long chains of many amino acids. All amino acids have the same basic structure.
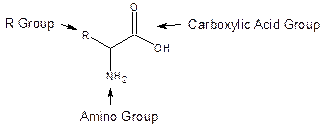
The amino group can be protonated and the carboxylic acid group can be deprotonated depending on the solution pH. There are over twenty different (biologically necessary) amino acids differing from one another by the atoms represented by R. In this experiment six different ones will be encountered (alanine, R=methyl; valine, R=isopropyl; leucine, R=isobutyl; phenylalanine, R=methylphenyl; aspartic acid, R= -CH2COOH; histidine, R=methylglyoxaline (cyclic ring structure, see below).
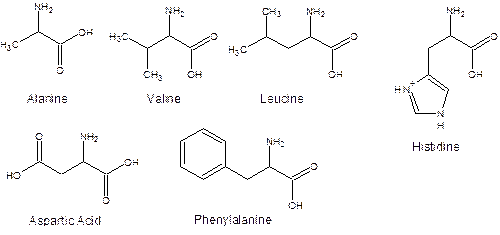
Individual amino acids can be combined by splitting out the water between the carboxylic acid group of the back amino acid and the amino group of the front amino acid.

The new bond formed between the carbonyl carbon atom and amino nitrogen atom is called a peptide bond and amino acids bonded together in this manner are called peptides. Two amino acids bonded together are referred to as a dipeptide, three a tripeptide, etc. A protein, however, is a polypeptide of molecular weight in the thousands. The properties possessed by a protein depend upon its size (i.e., the number of amino acids present), which amino acids are present (i.e., which R groups are present), and the sequence in which they appear within the chain. Thus, the analysis of any sample of protein forces the biochemist to be concerned with all three of these modes of its diversity.
An unknown containing an amino acid or mixture of amino acids will be tested to determine if alanine, leucine, valine, and/or histidine are present. These amino acids are separated based on differences in their “R” groups. Thin-layer chromatograph will be used to determine which of the four amino acids are present in the unknown for this experiment.
Aspartame, an artificial sweetener found in many diet drinks, is the methyl ester of the dipeptide aspartylphenylalanine (aspartic acid and phenylalanine). When hydrolyzed with HCl aspartylphenylalanine breaks apart into aspartic acid, phenylalanine, and methyl alcohol. Since methyl alcohol is toxic, opponents to approval by the Food and Drug Administration (FDA) claimed that aspartame used as a sweetener in food products would hydrolyze during storage creating a possible health hazard. The FDA ruled that aspartame was stable enough and approved its use in food products. An optional part of this experiment will allow you to test diet soda to see if any aspartic acid or phenylalanine are present indicating that the aspartame did indeed hydrolyze.

Aspartame
Thin-Layer Chromatography (TLC)
Thin-layer chromatography is a sensitive, fast, simple and inexpensive analytical technique. It is usually a qualitative measure of the makeup of a mixture, although in some instances it can be used quantitatively, or on a preparatory scale. Common uses of TLC include determination of the number of components in a mixture, determination of the identity of compounds in a mixture, monitoring the progress of a reaction, and effectiveness of purification. In this experiment we will use TLC to identify the components in a commercial analgesic and the amino acids in an unknown. The separation of compounds in TLC is based on relative solubility in a stationary and mobile phase, the same as other forms chromatography such as gas, liquid, or column chromatography. Three steps are involved in TLC: spotting, developing, and visualizing.

The TLC process involves spotting the sample to be analyzed along with possible known constituents on a sheet of glass, plastic or aluminum coated with a thin layer of an absorbent material. The most common absorbent material, and the one that we will use, is silica gel (SiO2·xH2O) is good for more polar compounds. Alumina (Al3O3) is another stationary phase better for less polar compounds.
The spotted plate is then developed by placing it on end in a solvent. The solvent is wicked up the plate by capillary action and in doing so carries the constituents of the mixture along with it. However, not every constituent travels the same amount. The distance that a compound travels is greatly determined by the polarity of the eluting solvent (mobile phase), absorbent material (stationary phase), and the constituent. Materials that more closely match the polarity of the eluting solvent will travel further than those that more closely resemble the polarity of the absorbent material. We will test various combinations of ethyl acetate and hexane (and perhaps other solvents) to determine the best one for separation of analgesics. A different solvent will be used as the mobile phase to develop or separate the amino acids.
The spots will be visualized using an ultraviolet (UV) light for the analgesics while the amino acids will be reacted with ninhydrin. This is done by spraying the plate with a ninhydrin solution which reacts with amino acids to form a colored complex. Spots containing each amino acid become visible upon heating; that is, a colored complex of the ninhydrin and amino acid is produced upon development by heat.
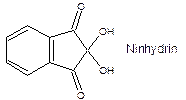
Iodine can also be used to visualize spots in some cases.
To correctly identify components of a mixture when compared with possible known components, a ratio between the distance traveled by solvent and component must be made. This ratio is called the retention factor (Rf ). This value will be the same for all components with the same identity. However, this value is system specific and most likely will change if a different solvent or if a different absorbent material is used. To calculate the Rf value, first measure the distance that the middle of the spot traveled and divide this value by the distance that the solvent front traveled. This is illustrated in figure 1.
Solvent (mobile phase) polarity with be very important in this experiment. The mobile phase could be pure or a mixture of solvents to obtain the best separation. The following is a list of solvents and their relative polarity:
|
Name |
Structure |
|
|
cyclohexane |
|
Least Polar |
|
hexane |
|
|
|
toluene |
|
|
|
dichloromethane |
CH2Cl2 |
|
|
ethyl acetate |
|
|
|
1-butanol |
|
|
|
ethanol |
|
|
|
methanol |
CH3OH |
|
|
water |
|
Most Polar |
EXPERIMENTAL PROCEDURE
Part I – Analysis of Commercial Analgesics[2]
Equipment, Materials, and Chemicals Needed:
§ UV light (eye hazard, do not view directly)
§ Pipettor 0.1 mL
§ Beaker (400 mL)
§ Wide parafilm
§ 5x10 cm TLC strips (silica gel on plastic, florescent)
§ 10 mL hexane (2302)
§ 10 mL ethyl acetate (2402)
§ 5-10 mL of 50/50 ethanol/t-butyl methyl ether solution (2312)
§ TLC solvent for part II - 4 parts 1-butanol, 1 part glacial acetic acid, and 1 part distilled water (3224)
§ 1% ninhydrin solution in acetone (2423)
§ Analgesic in ethanol (3313) and amino acid in water (2011) known solutions
§ Commercial analgesic (3112)
CAUTION!! Ethyl acetate, ethanol, acetone, t-butyl methyl ether, and hexane are flammable and toxic. Keep away from sources of ignition such as flames. Acetic acid and the ninhydrin in acetone solution are corrosive and volatile so avoid contact. Do all work with these chemicals in a fume hood. Use protective equipment such as gloves when handling and wear goggles at all times. The UV light is an eye hazard so do not view directly.
Spotting:
1. Each group should obtain two 5x10 cm strip of silica gel TLC plate.. Hold the plates by the edges and avoid touching the silica gel side of the plates. With a pencil, lightly draw a straight line parallel to the bottom of the plate about 1 cm from the bottom on each plate (see. Figure 1)
2. With a pencil, lightly place six equally spaced marks along the line on one plate. In a few minutes each group will spot each plate with the five analgesics and caffeine standards. On the 2nd plate each group will spot their assigned commercial analgesic and the 6 standards after the first plate is done. You might use AA for acetylsalicylic acid, Ac for acetaminophen, I for ibuprofen, N for naproxen, P for phenacetin, and C for caffeine, U for the commercial analgesic to very lightly label your plates with pencil.
3. Each lab group can obtain about ten drops of each of the six standard solutions in labelled clean test tubes. (Or there may be may be spotting stations at the end of each bench). Obtain a small diameter capillary tubes for each solution (type with both ends open, not ones used for melting point).
4. Each group should spot the plates as noted in #2 above. The spots should be kept as small as possible (1mm and no bigger than 2mm if possible). You might practice making small spots on a piece of paper first. To load solution into a capillary tube just touch the end of the tube to the solution. It will partially fill by capillary action.
5. After the spots have dried view them under a UV light to make sure they are easily visible.
Developing:
6. We will now test the effect of solvent polarity to determine the solvent combination that provides the best separation of the analgesics. Each lab group will test a different solvent combination with their first plate (assigned by TA) and write their results on the board. The solvents to be tested are ethyl acetate, hexane, and mixtures of the two for the following percents by volume of hexane: 0%, 25%, 50%, 75%, and 100%. We will also test 100% water and 100% ethanol as solvents if there are enough students. A small amount of acetic acid will also be added to each solution so each has about 1% acetic acid to maintain a constant pH.
7. First obtain a large square of parafilm.
8. Each group should prepare about 10 mL of the solvent to be tested using a 10 mL graduated cylinder and add 0.10 mL of glacial acetic acid to it using a pipettor in a fume hood (or add about 3 drops). Place the solution into a 400 mL beaker and cover with parafilm to reduce evaporation The solvent should be about ¼ cm deep and cover the bottom of the beaker. Keep the beaker in a well vented area (away from the edge of the lab bench).
9. Place the plate into the beaker with the shiny side facing the walls of the beaker. Make sure that the level of the eluting solvent does not go above the spotting line. If it does you get to start over. Cover the beaker with the parafilm and wait 6-10 minutes for the plate to develop. Do not move the beaker while the plate is being developed. The solvent front should travel 4-6 cm.
10. Remove the plate and immediately mark where the solvent front stopped with a pencil. Dispose of used hexane and ethyl acetate in the waste container labeled “Acetone Waste”.
Visualizing:
11. Allow the plate to thoroughly dry. View and mark the spots (with pencil) under a UV lamp.
12. Calculate the resulting Rf values for each spot and write them on the board for each solvent tested. Decide upon the solvent that gives the best separation and use that solvent to develop the second plate. Note all information in your lab notebook.
Analysis of Commercial Analgesic:
13. Crush the analgesic tablet provided by the TA in a mortor and pestle. Place the mixture in a small beaker and add 5-10 mL of a 50/50 mixture of ethanol and t-butyl methyl ether. On a stirrer/hot plate stir for 1-2 minutes and let settle. Filter if needed. Spot the solution along with the standards on a second plate as was done above.
14. Now spot, develop, and visualize the 2nd plate using the best solvent and calculate the Rf values for all the spots present and identify the components in the commercial analgesic.
15. Dispose of used hexane and ethyl acetate in the waste container labeled “Acetone Waste”.
Part II – Analysis of Unknown Amino Acid Solution (Individual Work Only):
Spotting the Samples onto the Plate:
1. Spot a fresh plate as was done above in the following order: Unknown Histidine Valine Alanine Unknown Leucine. After spotting the plate as shown in figure 1, dry the spots.
Development of Chromatography Plate:
2. The solvent for separating amino acids is 4 parts 1-butanol, 1 part glacial acetic acid, and 1 part distilled water.
CAUTION: This TLC solvent is flammable and corrosive, avoid skin contact, use only in a fume hood, and keep away from open flames.
Place about 10 mL in a clean 400 mL beaker so it is about three millimeters deep.
3. Place the silica gel plate spotted end down into the solvent in the beaker. Do not rock or move the beaker at this point! Make sure the solvent level in the beaker is well below the spots on the plate. Cover the beaker with a square piece of parafilm or aluminum foil and allow the solvent to climb up the plate for about 30 minutes. The solvent front should travel at least 4 cm. Note the length of time the plate(s) are in the beaker. The separation between spots (amino acids) will improve as the solvent front climbs farther up the plate, as long as it doesn’t go past the top of the plate.
4. Remove the plate when the solvent has moved far enough and mark the solvent front with a pencil line.
5. Allow the plate to dry in a vented location (fume hood). If needed place the plate in the oven to dry any remaining solvent (check the sheet every 30 seconds to make sure they don't turn brown).
6. Place the used TLC solvent in the container labeled “Acetone Waste” located in the hood.
Visualizing:
CAUTION: 1% Ninhydrin in ethanol spray is flammable and corrosive and will temporarily stain your hands. It should only be used in a fume hood wearing gloves.
7. Spray the silica gel on the plate with 1% ninhydrin spray, dry in the hood for a minute (to remove excess solvent), and heat in the oven for 1-3 minutes (do not let plate turn brown in oven, overheat). Remove and immediately circle the colored spots with a pencil. Note both the location and color of the spots.
8. Calculate the Rf for each spot and identify the amino acids in your unknown.
Part III: Analysis of aspartame in Diet Soft Drinks – Optional
Devise a procedure to determine if there is hydrolysis of aspartame in diet soft drinks using TLC. Check with your TA before attempting the experiment.
Followup:
Tape TLC strips into your lab notebook. Note the lab group and class Rf data in your notebook Using your data write a short statement on each of the following:
1. For part I which solvent gave the best separation of the analgesics? Why did the spot move with one solvent and not the other? Hint: Which solvent is least polar and which is most polar?
2. Explain how you identified the compounds in the commercial analgesic and the amino acid(s) in your unknown.
3. Relate the separation of the distance traveled by each of the amino acids tested to the R group and solubility in the mobile and stationary phase. What would be the pattern for aspartic acid and phenylalanine?
Fill out the unknown report sheet at the end of the experiment and turn in to the prep stockroom.
Pre-lab Questions:
1. What does TLC stand for?
2. Why do analgesic manufacturers add caffeine to their products?
3. What are some of the effects of caffeine on individuals? (lots of information on the web)
4. Draw the structures of caffeine, water, and dichloromethane.
5. What are the three parts of TLC analysis?
6. Why can’t the level of solvent in the beaker be higher than the spotting line on the plate?
7. Which is more polar, hexane or ethyl acetate? Why?
8. Calculate the Rf for a spot on a TLC plate if the solvent moved 10 cm and the spot moved 7 cm.
9. What is needed to view the spots for the analgesics on the TLC plates?
10. What is an amino acid?
11. What are three ways we can visualize spots?
12. Risk Assessment: What are the safety hazards and precautions for this experiment?
[1] Bettelheim and Landesberg, Experiments for introduction to Organic Chemistry: A Miniscale Approach. Saunders College Publishing, New York. © 1997. P. 57.
[1] Williamson, Kenneth L. Macroscale and Microscale Organic Experiments, 2nd Ed., D.C. Heath and Company, Lexington. © 1994. Pp. 161-162.
EXPERIMENT 7- Thin Layer Chromatography of Analgesics
and Amino Acids
CHM 235L REPORT SHEET FOR UNKNOWN AA-XXXX
STUDENT'S NAME____________________ Dana ID______ UNKNOWN # AA - _______
SECTION LETTER_____ LOCKER #_________ Workstation #________
TEACHING ASSISTANT_________________ INSTRUCTOR_______________________
This completed report sheet should be turned in to your TA before the end of the lab period. Do not put this sheet in the notebook. Do not write the hazard code, which has the form HC-xxxx, for the unknown number. The unknown number can be found on the vial containing the unknown or, if the vial is missing, the unknown number should also be recorded in the blue book in the prep stockroom.
Part I Data:
Solvent Percents* and Rf Values for first plate:
Hexane % ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Ethyl Acetate % ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Water % ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Ethanol % ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Caffeine ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
acetylsalicylic acid ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Acetaminophen ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Ibuprofen ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Naproxen ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
Phenacetin ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____
*About 1% Acetic Acid in each solvent.
Rf Values for spots on second plate: Solvent Used_______________________
Caffeine___ Aspirin___ Acetaminophen___ Ibuprofen___ Naproxen___ Phenacetin__
Commercial Analgesic ____ ____ ____ ____ ____ ____
Part II Data:
Rf Values for Alanine___ Valine___ Leucine___ Histidine___ Unknown____ ____ ____
Name of Amino Acid(s) in Unknown DP-xxxx: __________________________________
Teaching Assistant's Initials For Checking This Sheet____________________________
NOTE: This does not guarantee you identified your unknowns correctly