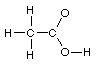
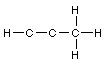
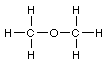
Total 100 points Name
1. Lewis structures. Add the remaining valence electrons to
complete the Lewis structures for the following skeletons. Avoid
formal charges.
![]()



2 Formal charges. Calculate the formal charge for all the atoms
other than H:(Don't draw any more electrons)
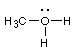
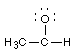
![]()
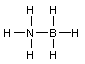
3 Resonance. Draw a resonance form for the following:
(a) ![]()
(b) 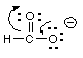
![]()
4 Molecular structure:
Predict the bond angles and hybridization of asterisked (*) for the molecule below:
![]()
(a) /_ H-C*-C (b) /_C-C*-N (c) /_C-N*-H .
hybridization:
.
(b) Circle all the cyclohexane rings 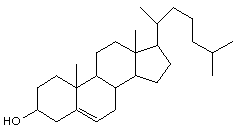
cholesterol
(c) What is the molecular formula for a linear alkane that has
25 C's?
(d) What is the molecular formula for a cyclic alkane that has 12 C's?
5 Functional groups.
Circle and identify the functional groups in the next two structures:
(a) 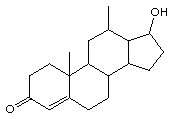 (b)
(b) 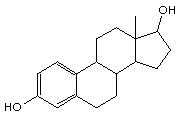
testosterone estradiol
6 Draw proper organic molecules (correct bonds), which contain
the following functional groups:
(a) alcohol (b) amide (c) ester (d) aldehyde
Draw a proper organic molecule that has
(a) one 2°C and two 1°C (b) a 4° carbon
7 Which compounds have the highest boiling points? Why?
(a) 3-methylpentane or n-hexane
(b) octane or propane
8 When hydrocarbons CnHm are combusted for heat or energy, what
are the products?
One of these products is thought to cause environmental danger?
Which product? What is the danger?
9 Name the following structures:
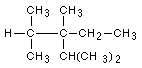
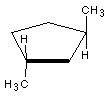
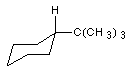
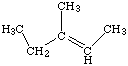
9b Draw the following molecules:
(a) 3-heptyne (b) 2-ethyl-3-propyl-3-heptene
(c) cis-1,4-dimethylcyclohexane
(c) sec-butylcyclopropane (d) 4-isopropyloctane
(e) E-1-bromo-2-chloro-1-pentene
10 Name and draw the Newman projections important conformations
of propane. Which one is less stable and why?
Draw the most and least stable chair forms of 1,2-dimethylcyclohexane.
Label most and least stable.