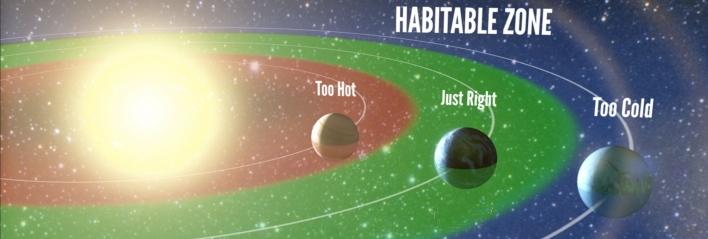
Water is one of the most common substances we interact with, so we tend not to think about it, and how special it is. Water is an important molecule with some unusual properties. Without liquid water, there could be no life as we know it. Life is possible on Earth because it exists in the so-called Goldilocks Zone, where it's neither too hot, nor too cold, but just right for liquid water to exist. In our solar system, the only other places where liquid water may exist in large volumes is under the ice of the moons of Jupiter and Saturn, where hydrothermal vents may allow chemosynthesis to occur.

Planet Ocean: Approximately 71% of the Earth's surface is covered in water, and most of that is ocean (salt water). Adult humans are about 65% water too! The origin of Earth's water is not completely understood, but much of the water is likely to have come from extra-terrestrial sources. Ice caps exist at both poles because they receive less solar radiation than equatorial regions, and the ice caps have a significant effect on both climate and sea level. There is growing evidence that the ice caps are shrinking as a result of human caused global climate change. The long-sought Northwest Passage has become navigable for at least part of the year for the first time in recorded history.
Tides: Gravitational interaction between the Earth's water and the Moon results in daily high and low tides. Tidal extremes were much greater in the Earth's early history, when the Moon was half as distant as it is now, and there is speculation that tides may have initiated or at least accelerated the evolution of life on Earth.
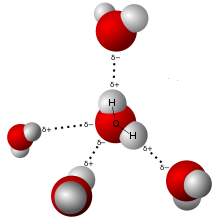
Water molecules attract each other due to polarity.
Polarity: Although the net charge of a water molecule is zero, water is polar because of its shape. The hydrogen ends of the molecule are positive and the oxygen end is negative. This causes water molecules to attract each other and other polar molecules. This attraction between water molecules can be seen on a smooth surface such as glass, where water beads up. The cohesion of water molecules to each other is an important part of the transpiration process in the xylem tissues of vascular plants. Water also has high surface tension because of this cohesion, and is the reason why water striders can skate on water.
Solvent: Water is sometimes called the universal solvent because it can dissolve a wide variety of chemicals. Acid-base chemistry depends on water. Crystalline salts dissociate in water to form positive and negative ions.
Metric Temperature: Since water is so important to life, the metric temperature scale was designed with 0 degrees as the freezing point of water, and 100 degrees as the boiling point of water (at sea level).
Density: An unusual characteristic of water is that, unlike most solids, ice is less dense than liquid water. For this reason, ice cubes float in a glass of water rather than sink to the bottom of the glass. As temperatures drop, lake and ocean water freezes from the top down, and the ice layer floats on top, insulating water below it from further cooling. If ice was more dense than liquid water, lakes would freeze from the bottom up, which would be much less conducive to living things. Salt water is more dense than freshwater so, if there isn't a lot of stirring, freshwater can form a lens on top of the salt water. This is common where rivers empty into the ocean, or where freshwater seeps up from springs on the ocean floor. These density differences cause light to scatter at different angles, reducing visibility.
Specific Heat: Water has a very high heat capacity. This means that it retains heat for a long time relative to the air. One important result of this is that large bodies of water moderate temperature. Coastal areas have less extreme summer and winter temperatures than the interior of major land masses. Since land masses lose heat faster than water does, the land tends to be warmer during the day and cooler at night than the ocean, resulting in onshore breezes in the morning and offshore breezes in the evening. Warming and cooling of water can form convection currents in the oceans too.
Evaporative Cooling: As water evaporates off the surface of an object, the faster moving molecules, with more kinetic energy, escape as a gas, while the lower energy molecules, with less kinetic energy, remain as liquid. This lowers the temperature on the surface from which the liquid is evaporating. Sweating is a form of evaporative cooling. Evaporative cooling works best when the humidity is low, which is why swamp coolers get high ratings in Arizona but not in Florida.
Reflectivity: Snow reflects so much light that prolonged exposure can cause snowblindness and sunburn. Snow has an albedo of 0.8 to 0.9. Since most light is reflected by a snow covered surface, little heat is absorbed and melting is slowed. This process, which can prolong winters and perhaps even contribute to ice ages, is the opposite of the greenhouse effect, and the reason why your car's interior should be white if you live in Phoenix.
Erosion: Ice takes up more physical space than the same amount of liquid water. This is why, if you fill a container of water to the top and then freeze it, the container may burst. This property of water is important to the erosion process. Water gets into cracks in rocks, freezes and expands. Cracks in the rock widen, letting more water in during the next thaw, and erosion continues. Over enough time, mountains can erode away though the repeated action of the freeze-thaw cycle.
https://www.youtube.com/watch?v=3jwAGWky98c
https://www.youtube.com/watch?v=z5Vm56Pu4hU
https://www.youtube.com/watch?v=h0py6BFlFZw